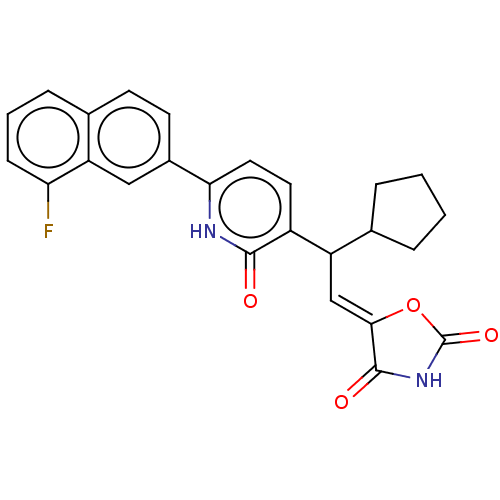
BDBM50553026 CHEMBL4794537
SMILES Fc1cccc2ccc(cc12)-c1ccc(C(\C=C2/OC(=O)NC2=O)C2CCCC2)c(=O)[nH]1
InChI Key InChIKey=GMYSSCNBOCLQGF-XKZIYDEJSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50553026
Found 2 hits for monomerid = 50553026
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataKi: 9nMAssay Description:Displacement of [3H]-PGE2 from human EP3 expressed in Chem-1 cell membranes incubated for 2 hrs by TopCount scintillation plate reader analysisMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP3 subtype(Homo sapiens (Human))
Janssen Research & Development
Curated by ChEMBL
Janssen Research & Development
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Antagonist activity at human EP3 expressed in CHO cells assessed as suppression of sulprostone-induced inhibition of forskolin induced cAMP productio...More data for this Ligand-Target Pair