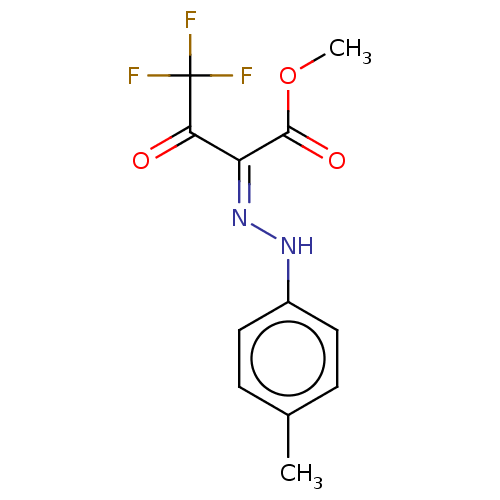
BDBM50570559 CHEMBL4868687
SMILES COC(=O)C(=N/Nc1ccc(C)cc1)\C(=O)C(F)(F)F
InChI Key InChIKey=WBTOVOOOKUVTED-MFOYZWKCSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50570559
Found 8 hits for monomerid = 50570559
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Competitive inhibition of porcine liver carboxylesterase by double reciprocal Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 1.01E+4nMAssay Description:Competitive inhibition of human erythrocyte AChE by double reciprocal Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataKi: 2.92E+4nMAssay Description:Competitive inhibition of equine serum BuChE by double reciprocal Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.83E+4nMAssay Description:Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's ...More data for this Ligand-Target Pair
TargetCholinesterase(Equus caballus (Horse))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of equine serum BuChE using butyrylcholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's methodMore data for this Ligand-Target Pair
TargetCocaine esterase(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 193nMAssay Description:Inhibition of human recombinant CES2 expressed in mouse NSO cells using 4-NPA as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase 1(Homo sapiens (Human))
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 16nMAssay Description:Inhibition of human recombinant CES1 expressed in baculovirus infected BTI insect cells using 4-NPA as substrate by spectrophotometric analysisMore data for this Ligand-Target Pair
TargetLiver carboxylesterase(Sus scrofa)
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Institute Of Physiologically Active Compounds Russian Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibition of porcine liver carboxylesterase using 4-NPA as substrate preincubated for 10 mins followed by substrate addition by spectrophotometric a...More data for this Ligand-Target Pair