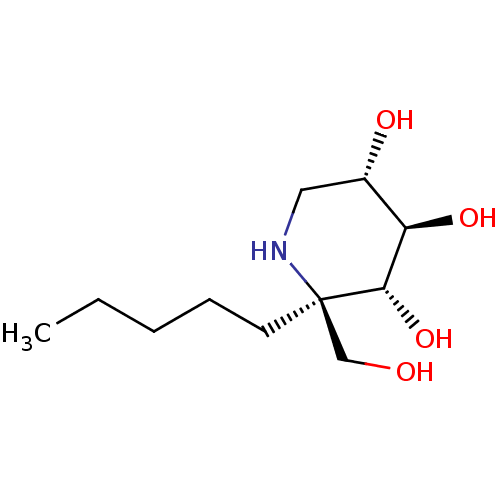
BDBM50583381 CHEMBL5029066::US20230339856, Compound (IIb1)
SMILES CCCCC[C@]1(CO)NC[C@H](O)[C@@H](O)[C@@H]1O
InChI Key InChIKey=ATZIJQARFQJARY-ZRUFSTJUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50583381
Found 3 hits for monomerid = 50583381
Affinity DataKi: 19nMAssay Description:Inhibition of human GAA using 4-methylumbelliferyl-alpha-D-glucopyranoside as substrate preincubated for 45 min followed by substrate addition and me...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nMAssay Description:The inhibiting activity on recombinant human acid α-glucosidase (rhGAA) of the compounds is implemented using the Fluopol-ABPP method (Fluoresce...More data for this Ligand-Target Pair
Affinity DataIC50: 4.91E+4nMAssay Description:The inhibiting activity on recombinant human acid α-glucosidase (rhGAA) of the compounds is implemented using the Fluopol-ABPP method (Fluoresce...More data for this Ligand-Target Pair