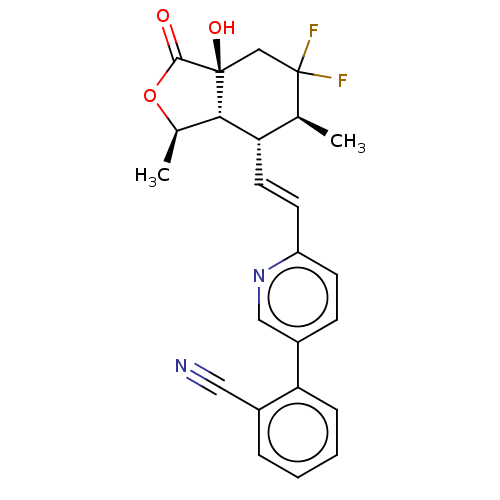
BDBM50585035 CHEMBL5084411
SMILES [H][C@@]12[C@@H](C)OC(=O)[C@]1(O)CC(F)(F)[C@@H](C)[C@@H]2\C=C\c1ccc(cn1)-c1ccccc1C#N
InChI Key InChIKey=RKRDXXXIGJTZKI-KJQHQIRPSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50585035
Found 2 hits for monomerid = 50585035
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
TBA
Curated by ChEMBL
TBA
Curated by ChEMBL
Affinity DataIC50: >6.00E+4nMAssay Description:Inhibition of [35S]MK-499 binding to hERG expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Antagonist activity at human PAR-1 expressed in HEK293 cells assessed as reduction in TRAP induced calcium signal at 3 uM by FLIPR analysisMore data for this Ligand-Target Pair