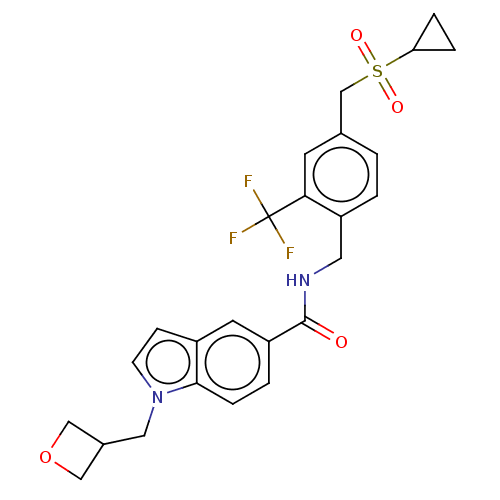
BDBM50586966 CHEMBL5091134
SMILES FC(F)(F)c1cc(CS(=O)(=O)C2CC2)ccc1CNC(=O)c1ccc2n(CC3COC3)ccc2c1
InChI Key InChIKey=HJQDFOLVXFVQLW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50586966
Found 2 hits for monomerid = 50586966
TargetPeroxisome proliferator-activated receptor gamma(Homo sapiens (Human))
Goethe-University
Curated by ChEMBL
Goethe-University
Curated by ChEMBL
Affinity DataEC50: 3.20E+3nMAssay Description:Partial agonist activity at GAL4-tagged human PPARgamma LBD expressed in HEK293T cells incubated for 12 to 14 hrs by dual-Glo luciferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of recombinant human full length sEH assessed as reduction in 6-methoxy-2-naphthaldehyde formation using PHOME as substrate preincubated f...More data for this Ligand-Target Pair