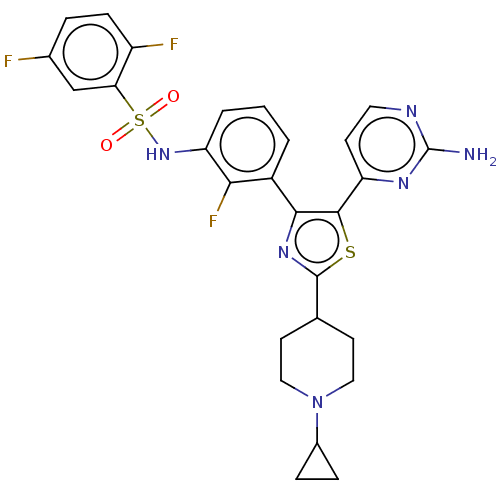
BDBM50602856 CHEMBL5183233
SMILES Nc1nccc(n1)-c1sc(nc1-c1cccc(NS(=O)(=O)c2cc(F)ccc2F)c1F)C1CCN(CC1)C1CC1
InChI Key InChIKey=GQNWRWISEOKRCC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50602856
Found 2 hits for monomerid = 50602856
Affinity DataEC50: 6.21E+3nMAssay Description:Transactivation of Gal4-tagged human PXR transfected in human HeLa cells preincubated for 16 hrs followed by luciferin addition and measured after 10...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Inhibition of N-terminal His-tagged human recombinant B-Raf V600E mutant (448 to 723 residues) expressed in Escherichia coli BL21 (DE3) by Lanthascre...More data for this Ligand-Target Pair