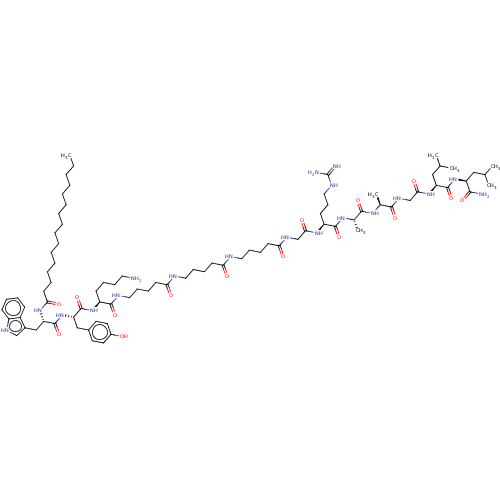
BDBM50603458 CHEMBL5196275
SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(=O)NCCCCC(=O)NCCCCC(=O)NCCCCC(=O)NCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CC(C)C)C(N)=O
InChI Key InChIKey=BRJKSKHPYACAGN-ZVIIWVDQSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50603458
Found 2 hits for monomerid = 50603458
Affinity DataIC50: 71nMAssay Description:Antagonist activity at human NPBWR1 expressed in CHO-RD-HGA16 cells measured by calcium mobilization assayMore data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at human NPBWR1 expressed in CHO-RD-HGA16 cells measured by calcium mobilization assayMore data for this Ligand-Target Pair