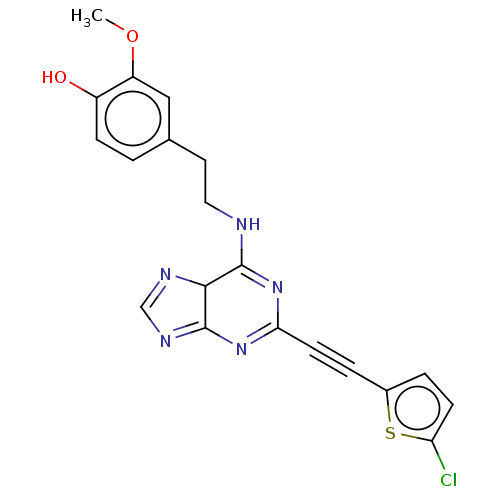
BDBM50603612 CHEMBL5171777
SMILES COc1cc(CCNC2=NC(=NC3=NC=NC23)C#Cc2ccc(Cl)s2)ccc1O
InChI Key InChIKey=MPFMHAAXLGXWPP-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50603612
Found 3 hits for monomerid = 50603612
TargetAdenosine receptor A3(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataKi: 6.82E+3nMAssay Description:Binding affinity towards human adenosine A3 receptorMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataEC50: 13nMAssay Description:Activation of human ABCG2-mediated ATPase activity preincubated for 2 mins followed by ATP addition and measured after 20 mins by colorimetric assayMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataIC50: 4.98E+3nMAssay Description:Inhibition of human ABCG2 in human R5 cells assessed as inhibition of mitoxantrone efflux measured by flow cytometric analysisMore data for this Ligand-Target Pair