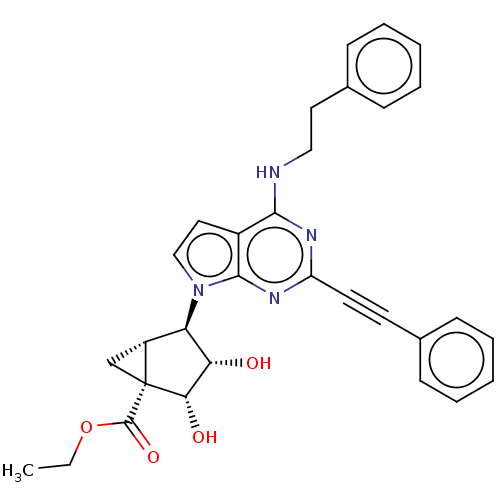
BDBM50603613 CHEMBL5169644
SMILES [H][C@]12C[C@@]1([C@@H](O)[C@@H](O)[C@@H]2n1ccc2c(NCCc3ccccc3)nc(nc12)C#Cc1ccccc1)C(=O)OCC
InChI Key InChIKey=ZPZDGGXNORFAFM-YOLPVHPBSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50603613
Found 10 hits for monomerid = 50603613
Affinity DataKi: 207nMAssay Description:Binding affinity to KOR (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 210nMAssay Description:Binding affinity towards human kappa opioid receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataKi: 483nMAssay Description:Binding affinity towards human adenosine A3 receptorMore data for this Ligand-Target Pair
TargetAdenosine receptor A3(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataKi: 483nMAssay Description:Binding affinity to human adenosine A3 receptor assessed as inhibition constantMore data for this Ligand-Target Pair
Affinity DataKi: 627nMAssay Description:Binding affinity to DOR (unknown origin)More data for this Ligand-Target Pair
TargetAdenosine receptor A3(Mus musculus)
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity towards mouse adenosine A3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to MOR (unknown origin)More data for this Ligand-Target Pair
TargetAdenosine receptor A3(Mus musculus)
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Binding affinity to mouse adenosine A3 receptor assessed as inhibition constantMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataEC50: 13nMAssay Description:Activation of human ABCG2-mediated ATPase activity preincubated for 2 mins followed by ATP addition and measured after 20 mins by colorimetric assayMore data for this Ligand-Target Pair
TargetBroad substrate specificity ATP-binding cassette transporter ABCG2(Homo sapiens (Human))
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
National Cancer Institute (Ba, Mm, Sl, Sva)
Curated by ChEMBL
Affinity DataIC50: 440nMAssay Description:Inhibition of human ABCG2 in human R5 cells assessed as inhibition of mitoxantrone efflux measured by flow cytometric analysisMore data for this Ligand-Target Pair