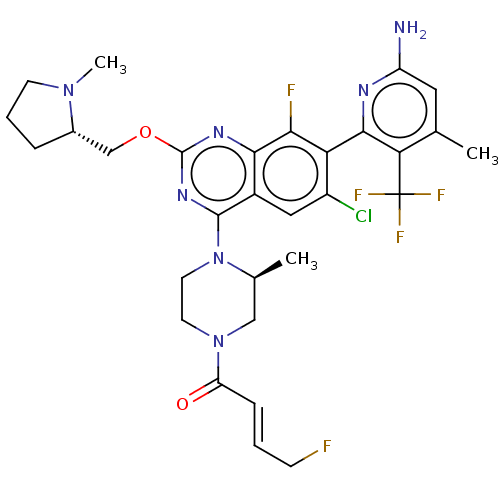
BDBM535155 US11236068, Example 18a::US11236068, Example 18b
SMILES C[C@H]1CN(CCN1c1nc(OC[C@@H]2CCCN2C)nc2c(F)c(c(Cl)cc12)-c1nc(N)cc(C)c1C(F)(F)F)C(=O)\C=C\CF
InChI Key InChIKey=GPOJTNHHDXMTFF-LETPOXDLSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 535155
Found 2 hits for monomerid = 535155
Affinity DataIC50: 960nMAssay Description:To determine the potency of compounds for inhibiting nucleotide exchange, various concentrations were incubated with K-Ras G12C (25 nM in reaction, 1...More data for this Ligand-Target Pair
Affinity DataIC50: 8nMAssay Description:To determine the potency of compounds for inhibiting nucleotide exchange, various concentrations were incubated with K-Ras G12C (25 nM in reaction, 1...More data for this Ligand-Target Pair