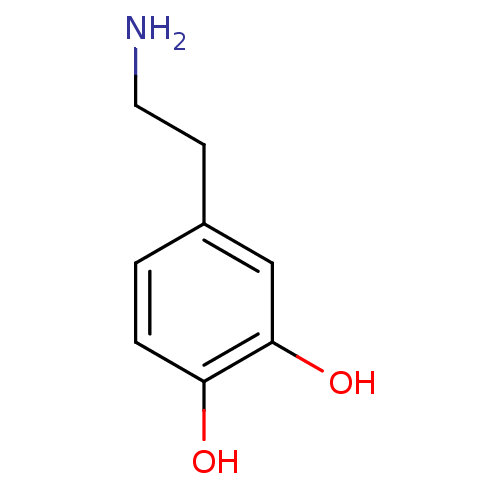
BDBM55121 3-HYDROXYTYRAMINE HYDROCHLORIDE::4-(2-aminoethyl)benzene-1,2-diol;hydrochloride::4-(2-aminoethyl)pyrocatechol;hydrochloride::4-(2-azanylethyl)benzene-1,2-diol;hydrochloride::Dopamine::MLS000069419::SMR000059081::cid_65340
SMILES NCCc1ccc(O)c(O)c1
InChI Key InChIKey=VYFYYTLLBUKUHU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 55121
Found 3 hits for monomerid = 55121
TargetD(2) dopamine receptor(Homo sapiens (Human))
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Agonist activity at human D2S receptor expressed in HEK293T cell membranes coexpressing Galphao1 assessed as induction of nucleotide exchange preincu...More data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Affinity DataEC50: 160nMAssay Description:Partial agonist activity at human D2SR expressed in HEK293T cells co-expressing (EA)beta-arrestin2 and GRK2 assessed as induction of beta-arrestin2 r...More data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Homo sapiens (Human))
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Friedrich-Alexander University Erlangen-Nuernberg
Curated by ChEMBL
Affinity DataEC50: 390nMAssay Description:Partial agonist activity at human D2SR expressed in HEK293T cells co-expressing (EA)beta-arrestin2 assessed as induction of beta-arrestin2 recruitmen...More data for this Ligand-Target Pair