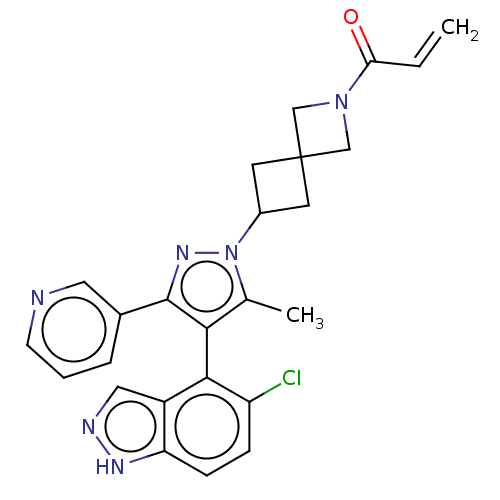
BDBM608868 US11702409, Example 19a::US11702409, Example 19b
SMILES Cc1c(c(nn1C1CC2(C1)CN(C2)C(=O)C=C)-c1cccnc1)-c1c(Cl)ccc2[nH]ncc12
InChI Key InChIKey=GPSWEICMVSMBQY-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 608868
Found 3 hits for monomerid = 608868
Affinity DataIC50: 1.90E+3nMAssay Description:Assays were run using 384-well plates (781207/Greiner) in which one column was designated as the high signal (no inhibition) control, and contained D...More data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of KRAS G12C mutant in human NCI-H358 cells assessed as reduction in ERK phosphorylation incubated for 6 hrs by MSD assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Assays were run using 384-well plates (781207/Greiner) in which one column was designated as the high signal (no inhibition) control, and contained D...More data for this Ligand-Target Pair