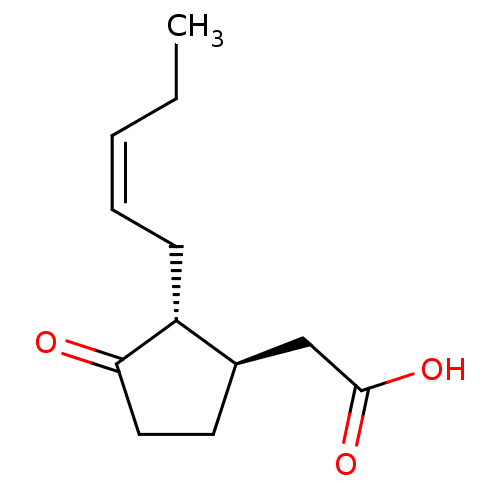
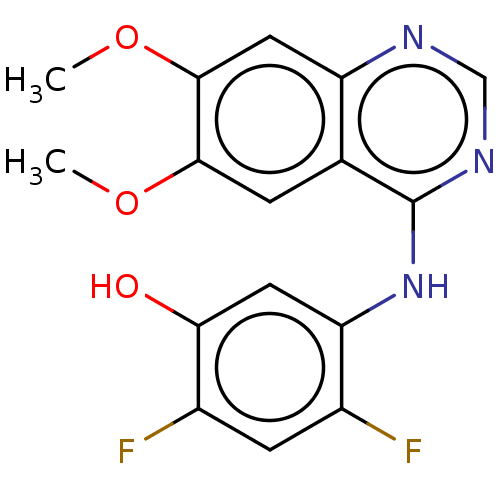
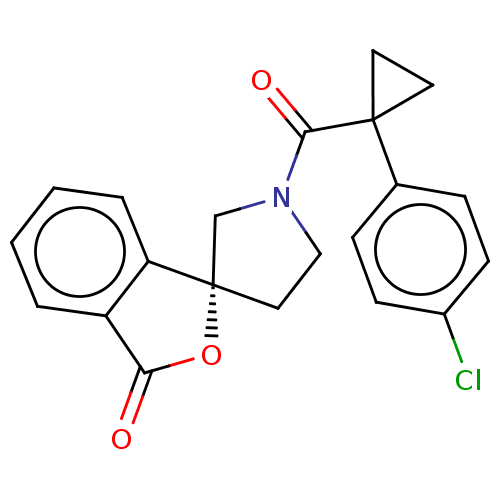
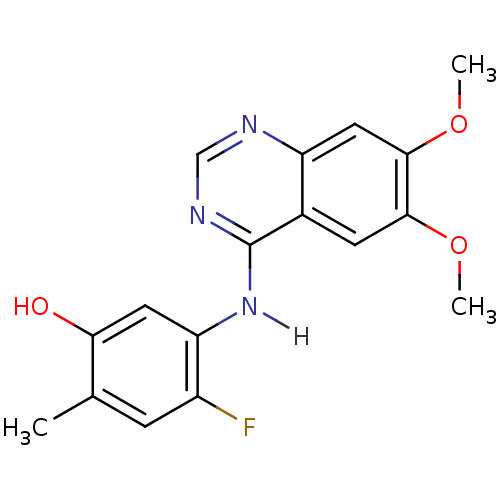
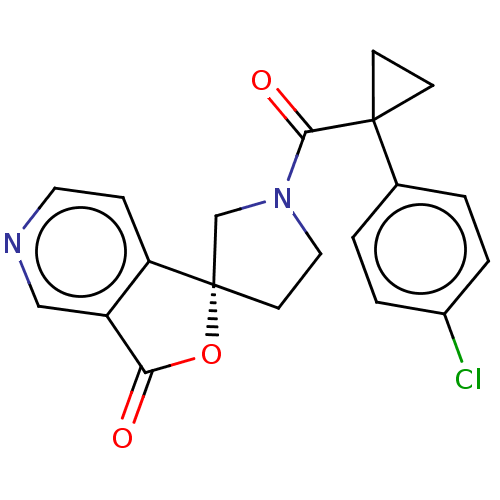
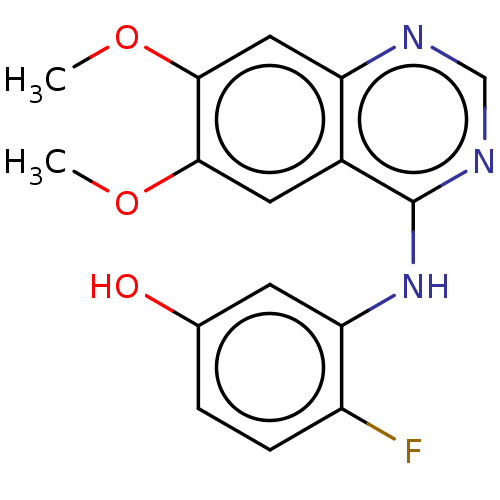
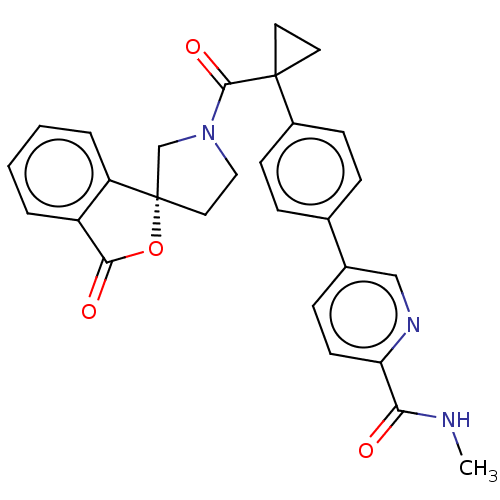
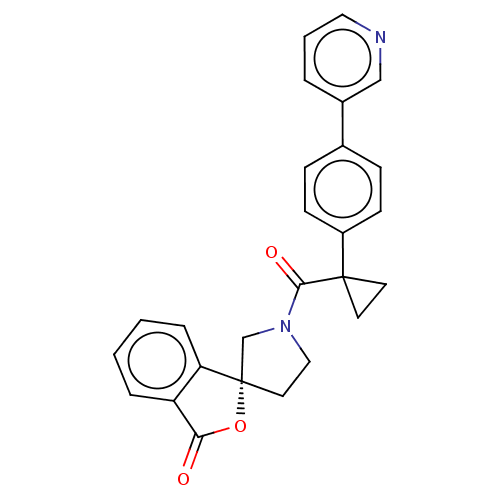
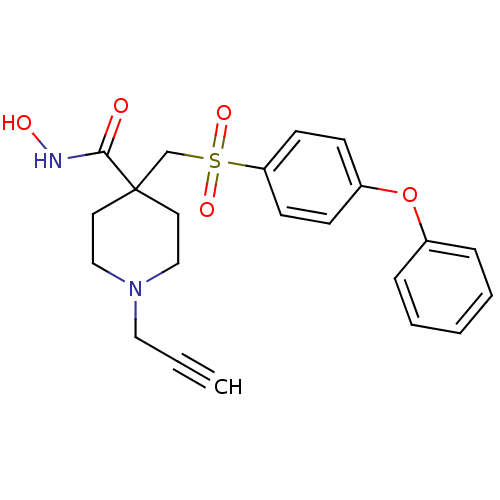
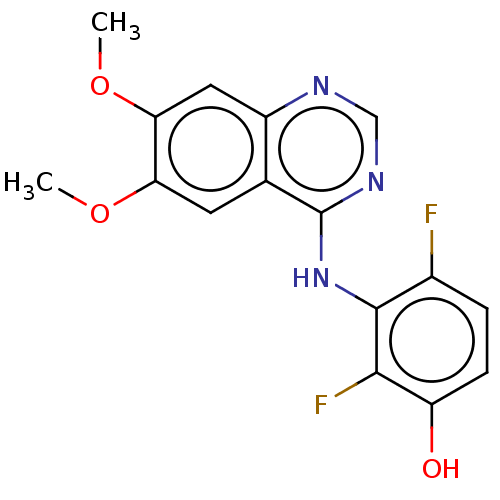
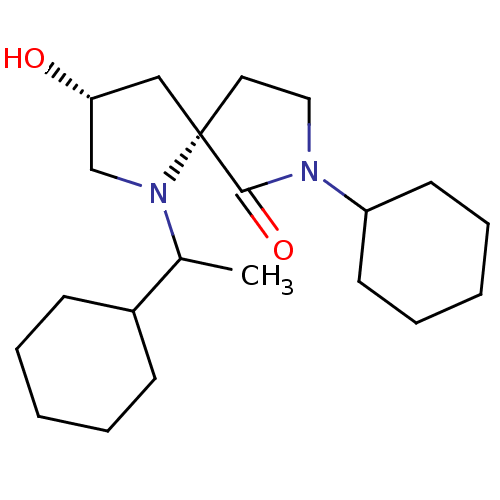
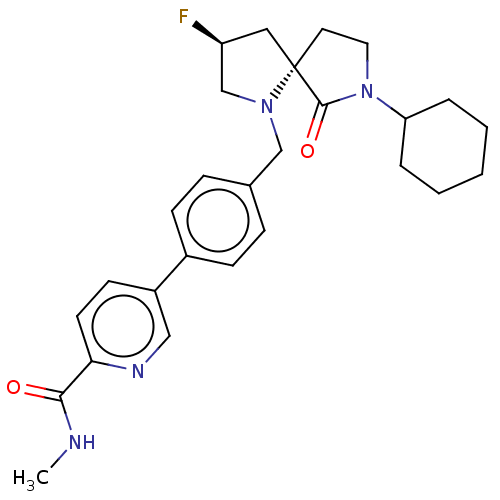
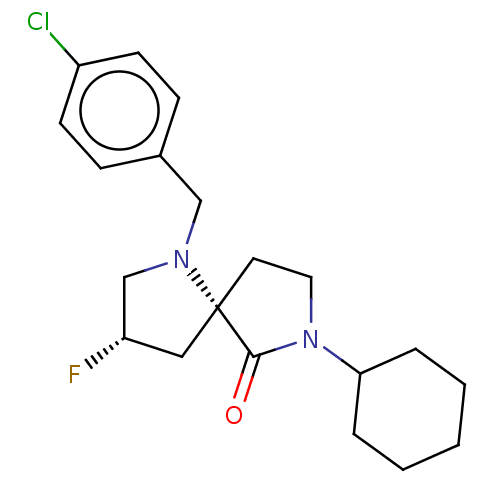
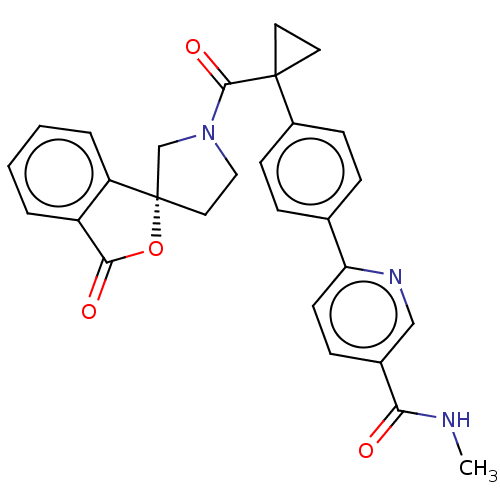
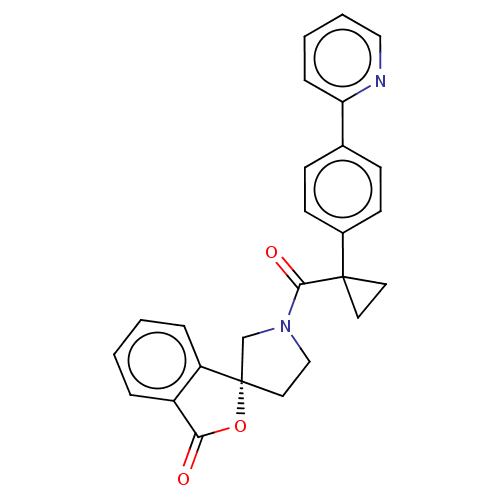
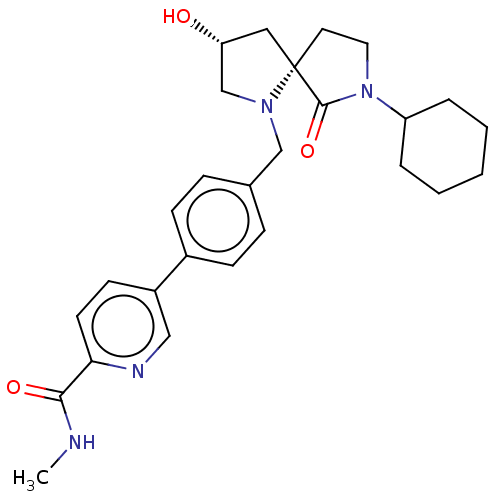
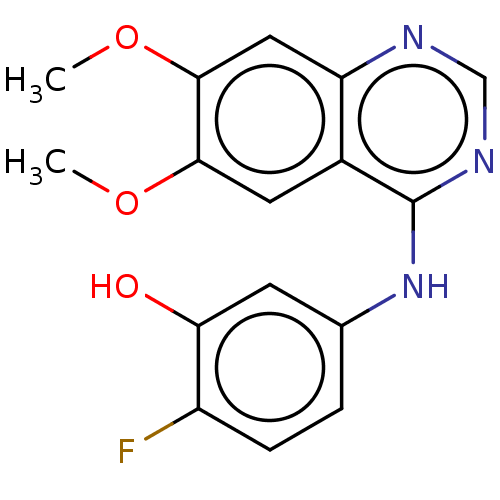
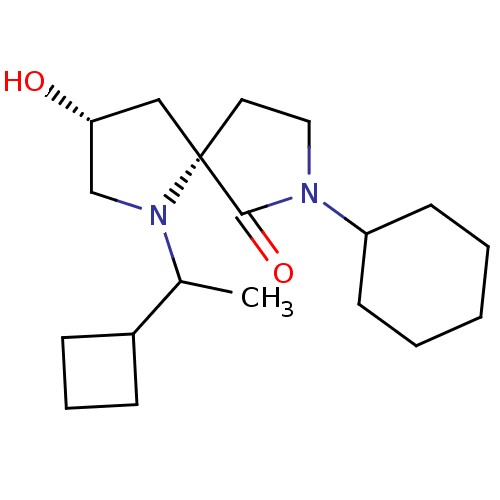
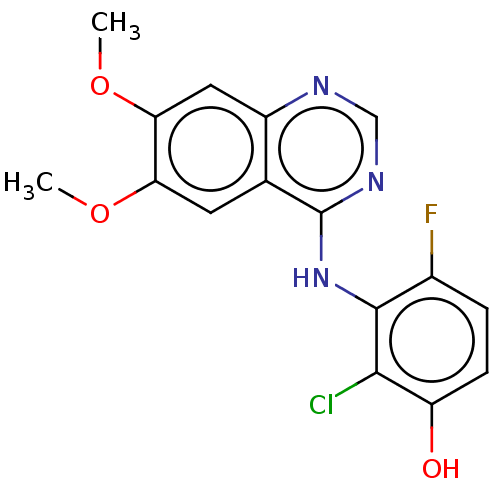
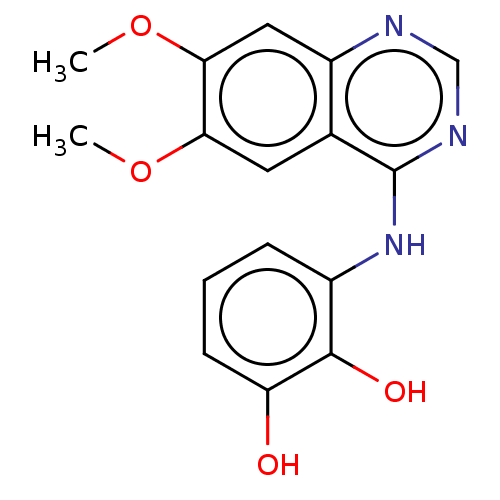
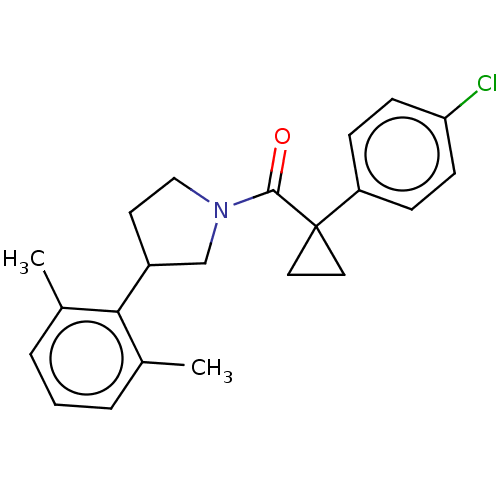
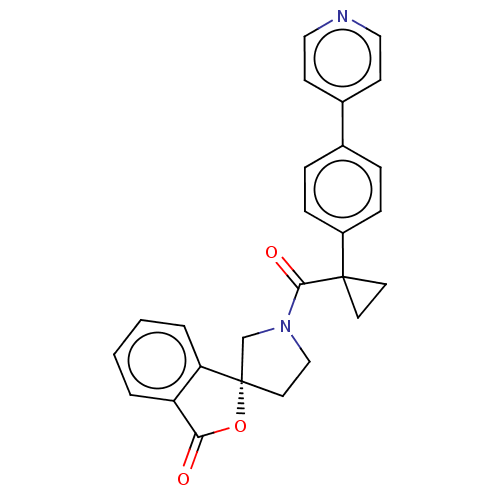
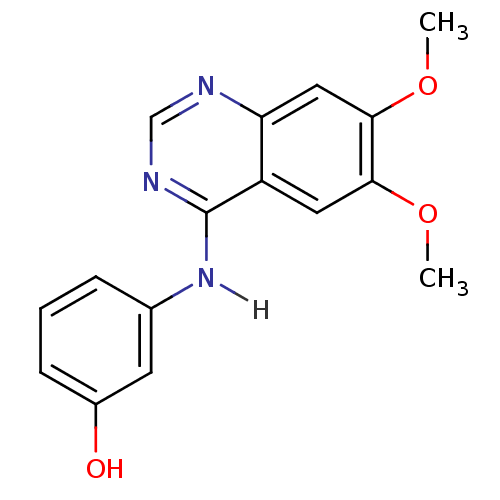
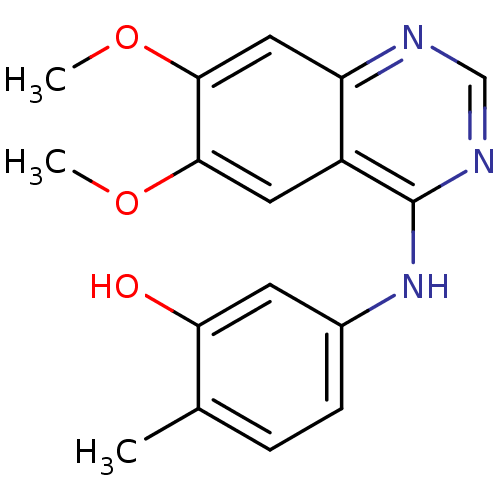
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 6.90nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
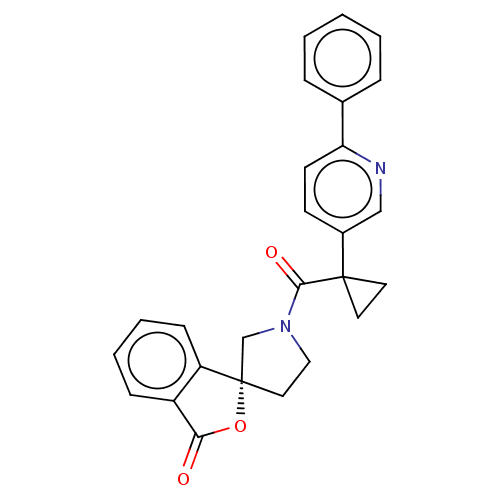
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 380nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
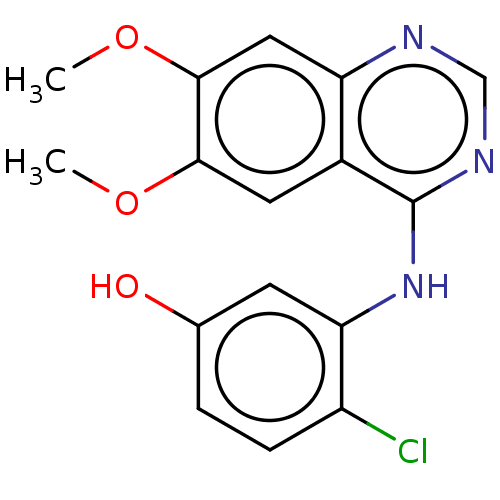
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 1.32E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
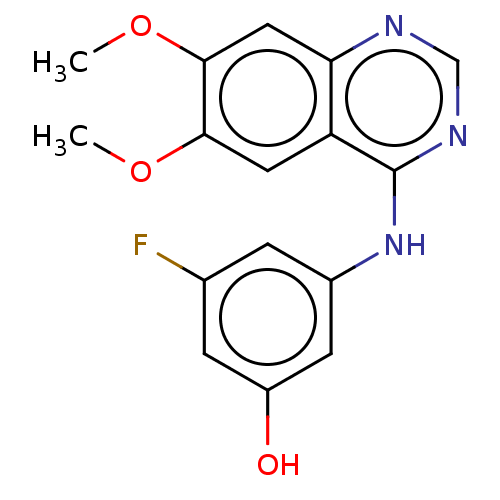
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 2.66E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 6.00E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 8.20E+3nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 1.50E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 2.10E+4nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: >1.00E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Homo sapiens (Human))
University of Pennsylvania
US Patent
University of Pennsylvania
US Patent
Affinity DataKi: 1.06E+5nMAssay Description:Compounds may be evaluated as selective reversible inhibitors of AKR1C3 by screening them against homogeneous recombinant AKR1C1-AKR1C4 expressed in ...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 0.410nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 0.75nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 1nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of 11beta-HSD1 in human PBMC cells using 3H-cortisone as substrate assessed as conversion of cortisone to cortisol by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin) using 3H-cortisone as substrate by scintillation proximity assayMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin) using 3H-cortisone as substrate by scintillation proximity assayMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.20nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.30nMAssay Description:Inhibition of recombinant His-tagged human KDR expressed in insect Sf21 cells preincubated for 15 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.40nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 2.60nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin)More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin) using 3H-cortisone as substrate by scintillation proximity assayMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin)More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.70nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of 11beta-HSD1 in human PBMC cells using 3H-cortisone as substrate assessed as conversion of cortisone to cortisol by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of 11beta-HSD1 (unknown origin)More data for this Ligand-Target Pair
Target11-beta-hydroxysteroid dehydrogenase 1(Homo sapiens (Human))
Incyte Research Institute
Curated by ChEMBL
Incyte Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of human11beta-HSD1 in human PBMC cellsMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
University Of Manchester
Curated by ChEMBL
University Of Manchester
Curated by ChEMBL
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human RET cytoplasmic domain (658 to 1114 residues) expressed in baculovirus system preincubated for 15 mins followed by substrate addi...More data for this Ligand-Target Pair

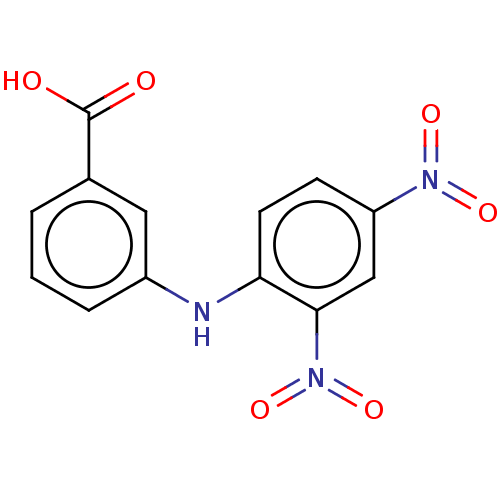
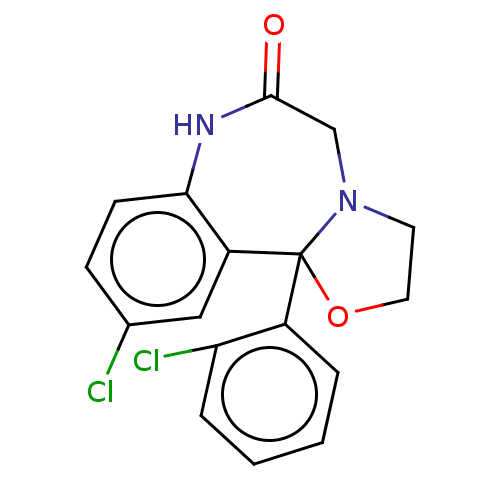
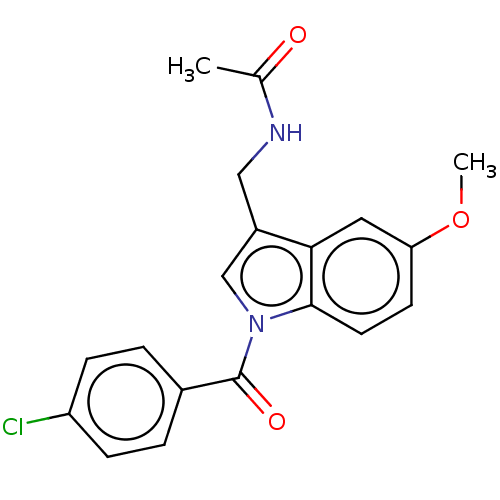
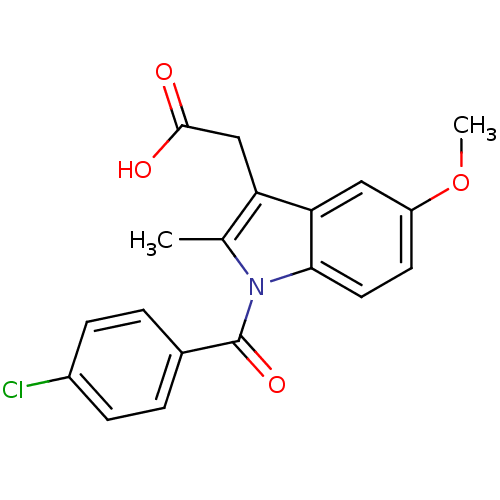
 3D Structure (crystal)
3D Structure (crystal)