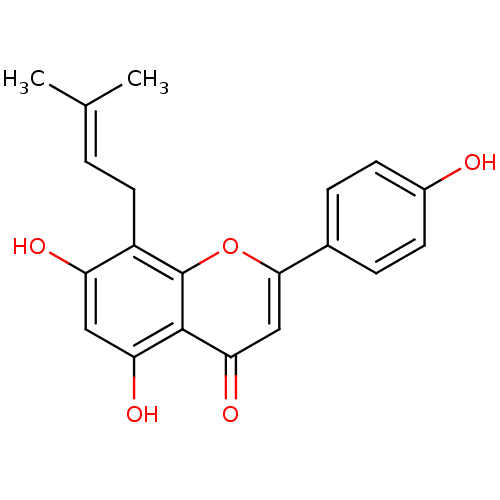
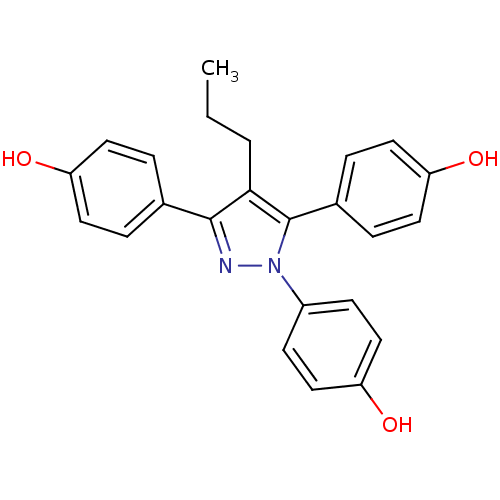
Affinity DataKi: 36nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Competitive inhibition of human recombinant CYP1A1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Competitive inhibition of human recombinant CYP3A5 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 203nMAssay Description:Displacement of [3H]-HU-243 from CB2 receptor (unknown origin) expressed in African green monkey Cos7 cell membranes incubated for 90 mins by radioli...More data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Mixed type inhibition of human recombinant CYP2B6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Mixed type inhibition of human recombinant CYP2C19 using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH-generating system add...More data for this Ligand-Target Pair
Affinity DataKi: 842nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes incubated for 90 mins by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Competitive inhibition of human recombinant CYP3A4 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of 5-HT2C (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of mu-type opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of kappa-type opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.42E+3nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 10 mins in presence of NADPH generating by Lineweave...More data for this Ligand-Target Pair
Affinity DataKi: 2.69E+3nMAssay Description:Competitive inhibition of human recombinant CYP1A2 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition of D1 dopamine receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.86E+3nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition of histamine H3 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of alpha2B receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of sigma2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.63E+3nMAssay Description:Competitive inhibition of human recombinant CYP1B1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of alpha2C receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.35E+3nMAssay Description:Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 5.60E+3nMAssay Description:Competitive inhibition of CYP2C9 in pooled human liver microsomes using S-warfarin as substrate preincubated for 5 mins followed by NADPH-generating ...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nMAssay Description:Inhibition of delta-type opioid receptor receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.23E+4nMAssay Description:Mixed type inhibition of human recombinant CYP3A7 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 30 mins f...More data for this Ligand-Target Pair
Affinity DataKi: 2.07E+4nMAssay Description:Competitive inhibition of CYP2C11 in rat liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:Non-competitive inhibition of human recombinant CYP2A6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 ...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 1.24E+5nMAssay Description:Mixed type inhibition of CYP17 in Sprague-Dawley rat testis microsomes assessed as reduction in 17alpha-Hydroxylase activity in presence of NADPH-gen...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 4.50E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Homo sapiens (Human))
Chinese Academy Of Sciences
Curated by ChEMBL
Chinese Academy Of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate after 3 mins by colorimetric analysisMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 4.76E+4nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.18E+5nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.18E+5nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: 1.27E+5nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of Illinois At Chicago
Curated by ChEMBL
University Of Illinois At Chicago
Curated by ChEMBL
Affinity DataIC50: >1.50E+5nMAssay Description:Displacement of [3H]LSD from human recombinant 5HT7 receptor expressed in CHO cells by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 400nMAssay Description:Estrogenic activity at estrogen receptor alpha in human Ishikawa cells assessed as induction of alkaline phosphatase activity using p-Nitrophenol pho...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Rattus norvegicus (rat))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataEC50: 3.20E+4nMAssay Description:Displacement of [3H] ketanserin from rat 5-HT2aR expressed in mouse NIH/3T3 cells incubated for 30 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+3nMAssay Description:Estrogenic activity at estrogen receptor alpha in human Ishikawa cells assessed as induction of alkaline phosphatase activity using p-Nitrophenol pho...More data for this Ligand-Target Pair
Affinity DataEC50: 0.200nMAssay Description:Estrogenic activity at estrogen receptor alpha in human Ishikawa cells assessed as induction of alkaline phosphatase activity using p-Nitrophenol pho...More data for this Ligand-Target Pair
Affinity DataEC50: 3.5nMAssay Description:Estrogenic activity at ERbeta (unknown origin) expressed in human MDA-MB-231/beta41 cells after 18 hrs by renilla luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 1nMAssay Description:Estrogenic activity at ERalpha in human Ishikawa cells assessed as induction of alkaline phosphatase activity using p-nitrophenol as substrate treate...More data for this Ligand-Target Pair

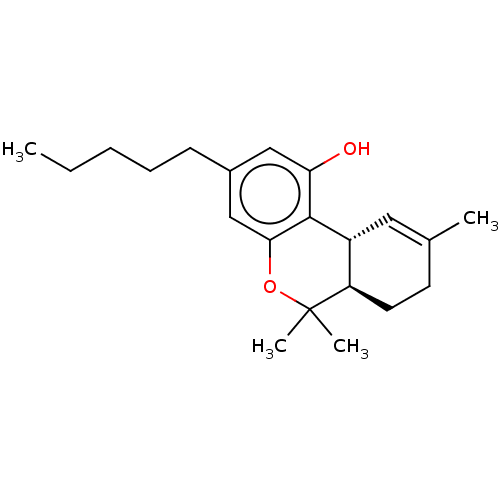
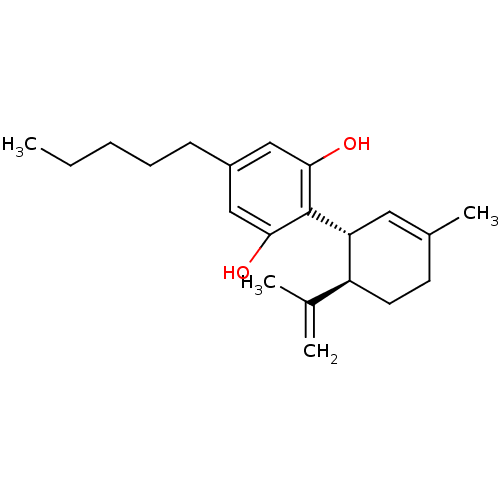
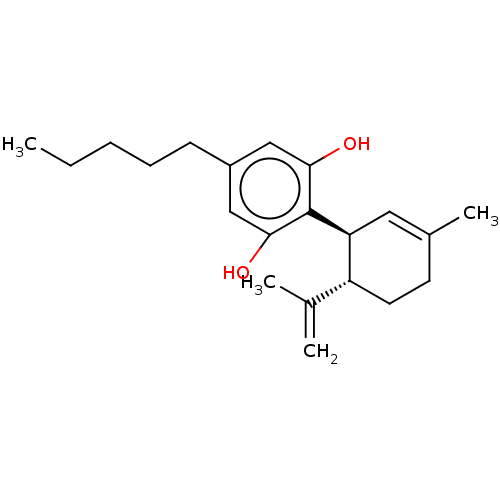

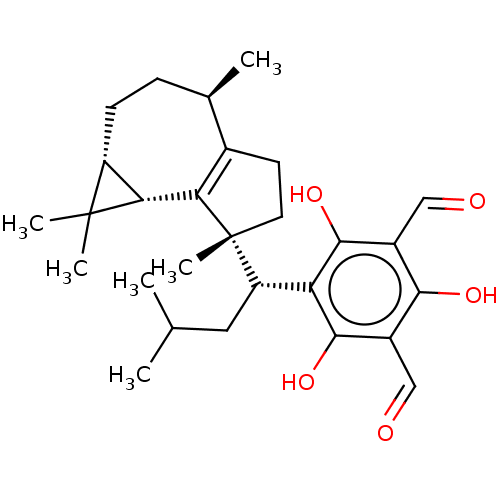

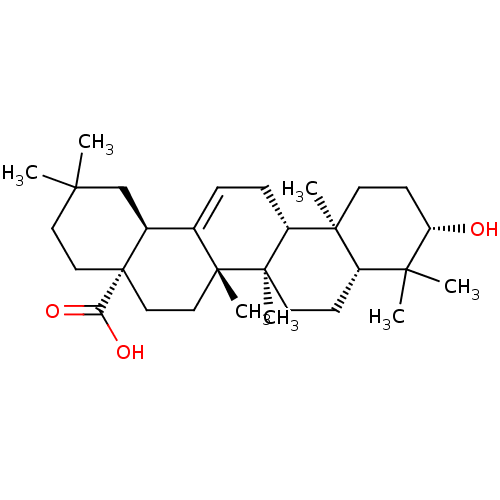

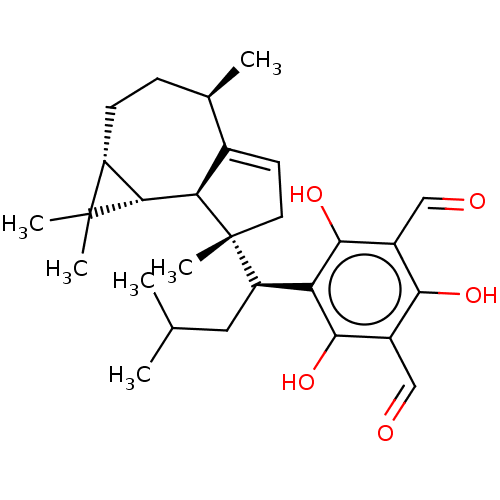
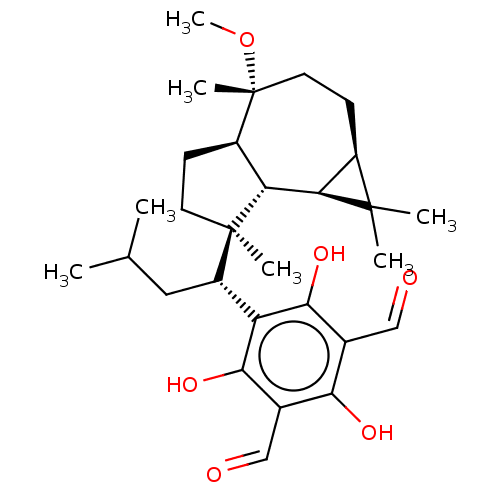
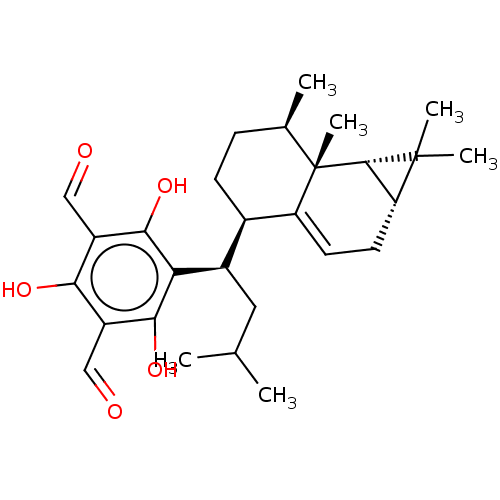
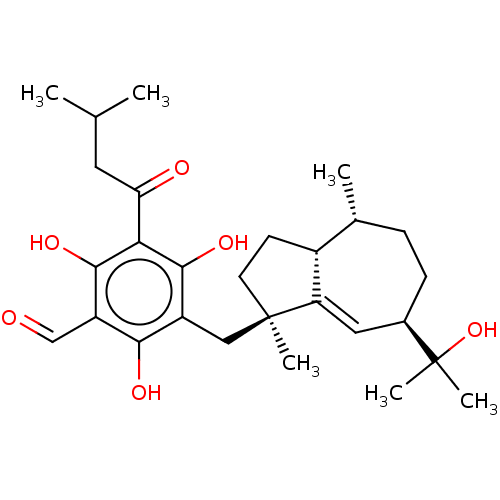
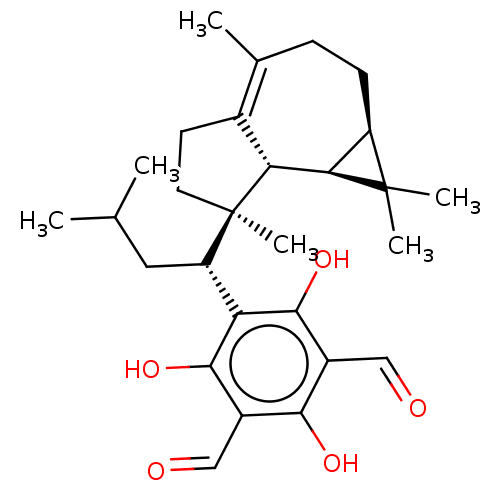
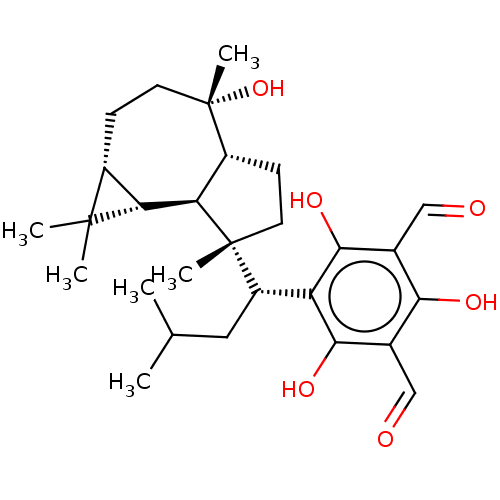
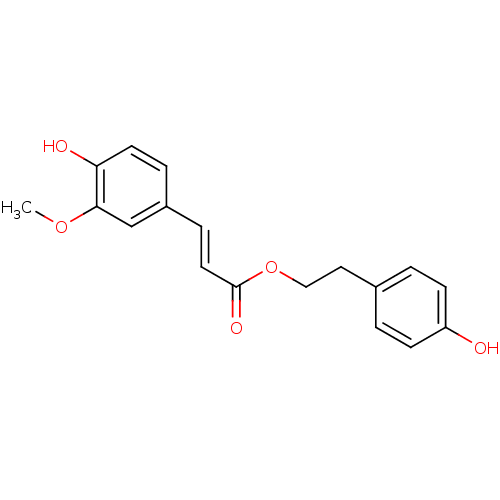
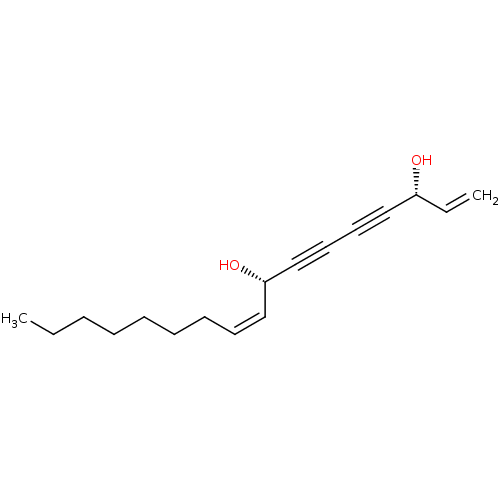
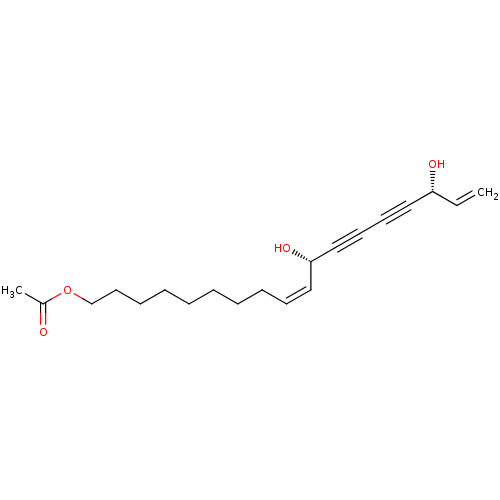
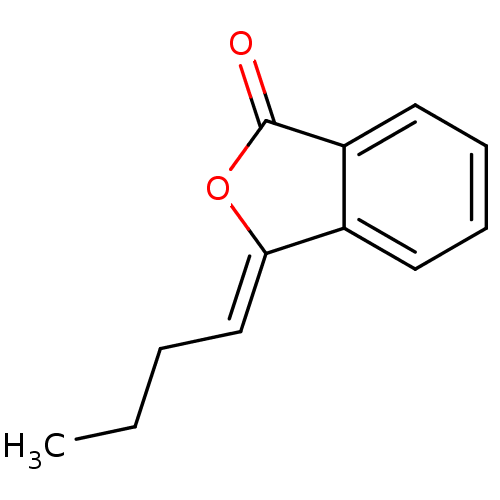

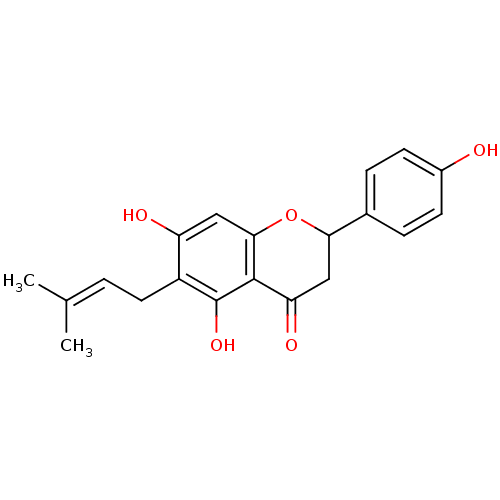
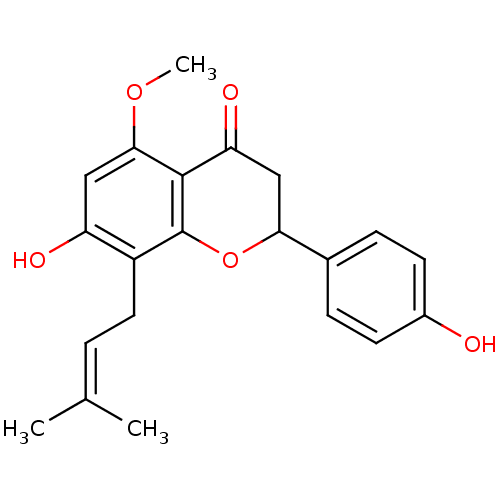
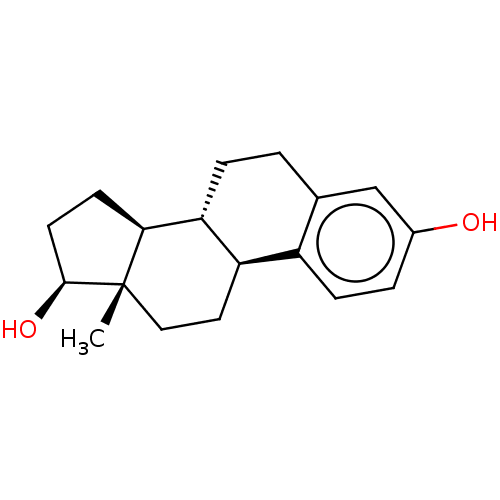
 3D Structure (crystal)
3D Structure (crystal)