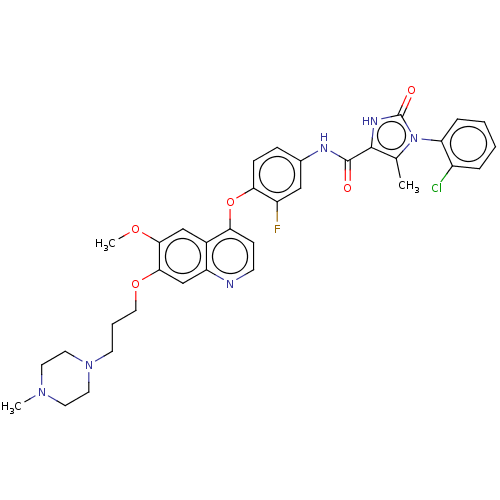
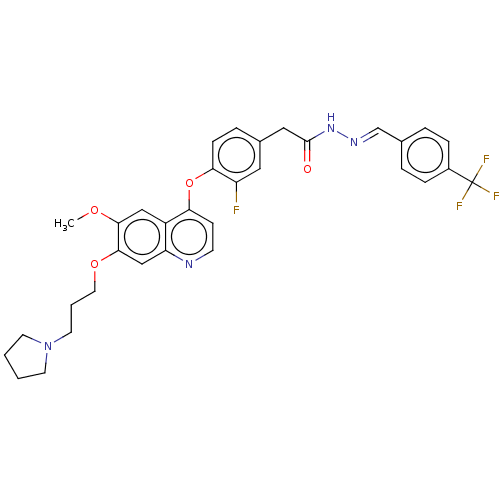
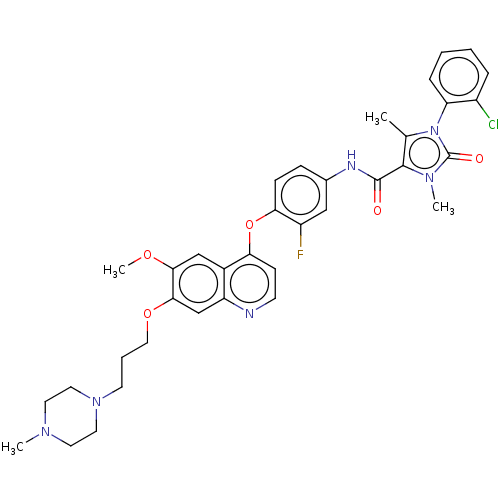
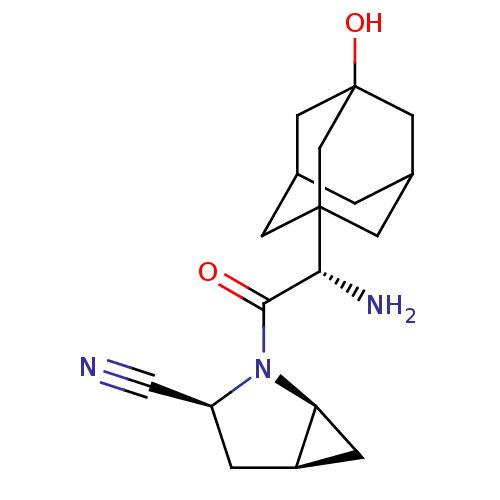
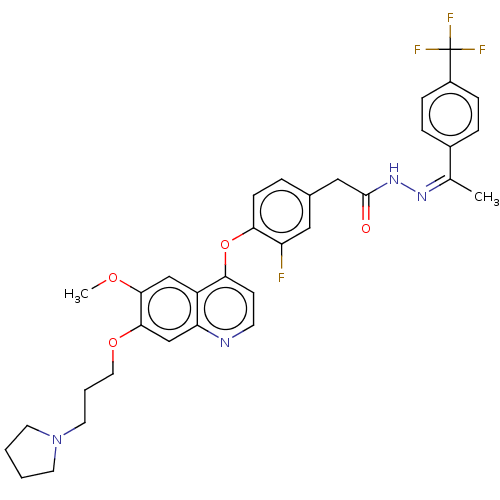
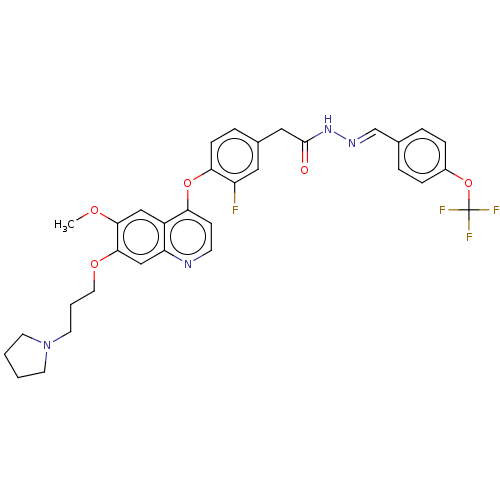
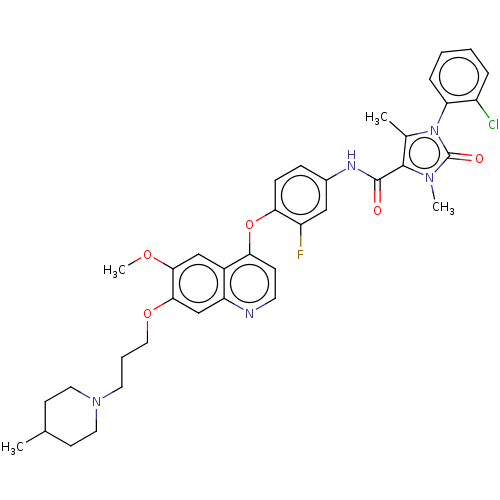
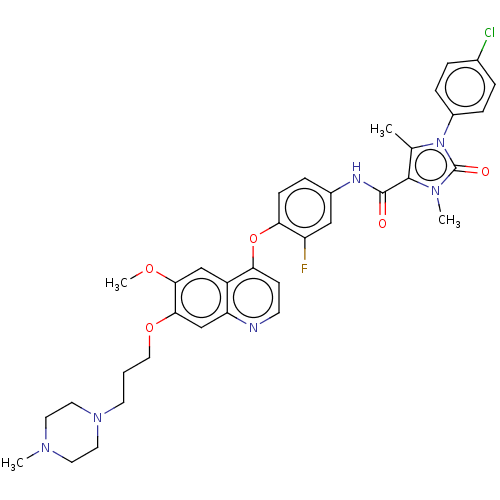
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Guizhou Medical University
Curated by ChEMBL
Guizhou Medical University
Curated by ChEMBL
Affinity DataKi: 5.10nMAssay Description:Inhibition of His-tagged human full length PI3Kalpha coexpressed with p85 alpha in baculovirus expression system using PIP2 peptide as substrate incu...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 33nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 39nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 43nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 46nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 48nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 57nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 66nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 83nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform(Homo sapiens (Human))
Guizhou Medical University
Curated by ChEMBL
Guizhou Medical University
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Inhibition of N-terminal His6-tagged human recombinant full length PI3Kbeta co-expressed with human recombinant full length P85alpha in baculovirus i...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 94nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 105nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 114nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 144nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 188nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 193nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Guizhou Medical University
Curated by ChEMBL
Guizhou Medical University
Curated by ChEMBL
Affinity DataKi: 193nMAssay Description:Inhibition of GST-tagged human recombinant PI3Kgamma catalytic domain (468 to 1203 residues) expressed in insect cells using PIP2 peptide as substrat...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 203nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Guizhou Medical University
Curated by ChEMBL
Guizhou Medical University
Curated by ChEMBL
Affinity DataKi: 245nMAssay Description:Inhibition of N-terminal His6-tagged human recombinant full length PI3Kdelta co-expressed with human recombinant full length P85alpha in baculovirus ...More data for this Ligand-Target Pair
TargetSignal transducer and activator of transcription 3(Homo sapiens (Human))
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
University Of Texas M. D. Anderson Cancer Center
Curated by ChEMBL
Affinity DataKi: 386nMAssay Description:Binding affinity to SH2 domain of Stat3 by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 1nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 1nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 substrate by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 substrate by HTRF assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 2nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 2.90nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 3nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 3nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 3nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 3nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant DPP4 by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of human recombinant DPP4 by fluorescence assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 3.10nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 substrate by HTRF assayMore data for this Ligand-Target Pair
TargetMacrophage-stimulating protein receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of Ron (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetMacrophage-stimulating protein receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 3.40nMAssay Description:Inhibition of RON (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Guizhou Medical University
Curated by ChEMBL
Guizhou Medical University
Curated by ChEMBL
Affinity DataIC50: 3.90nMAssay Description:Inhibition of His-tagged human full length PI3Kalpha coexpressed with p85 alpha in baculovirus expression system using PIP2 peptide as substrate incu...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 4nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 4nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 substrate by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetHepatocyte growth factor receptor(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 4.40nMAssay Description:Inhibition of c-Met (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of VEGFR-2 (unknown origin) using poly (Glu, Tyr) 4:1 as substrate after 30 mins by HTRF assayMore data for this Ligand-Target Pair
TargetVascular endothelial growth factor receptor 2(Homo sapiens (Human))
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Key Laboratory Of Structure-Based Drug Design And Discovery (Shenyang Pharmaceutical University)
Curated by ChEMBL
Affinity DataIC50: 4.90nMAssay Description:Inhibition of KDR (unknown origin)More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 5nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 5nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Homo sapiens (Human))
Zhejiang Hisun Pharmaceutical
US Patent
Zhejiang Hisun Pharmaceutical
US Patent
Affinity DataIC50: 5nMAssay Description:he experimental process was briefly described as follows: Test compounds were first dissolved in DMSO to prepare storage solutions. The reaction was ...More data for this Ligand-Target Pair


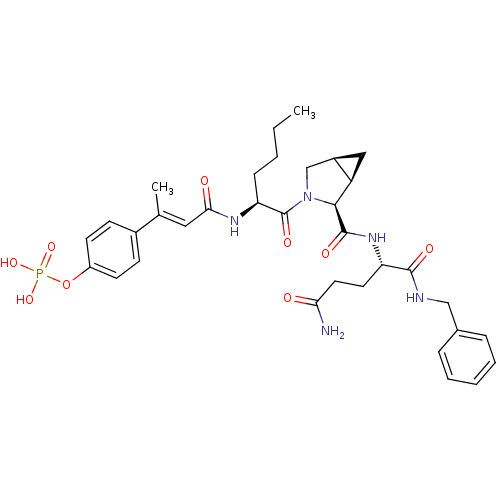
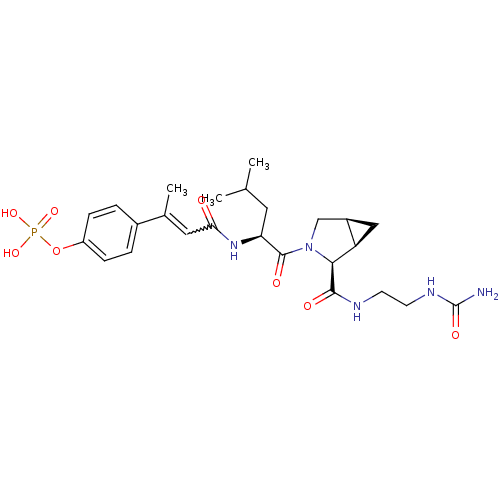
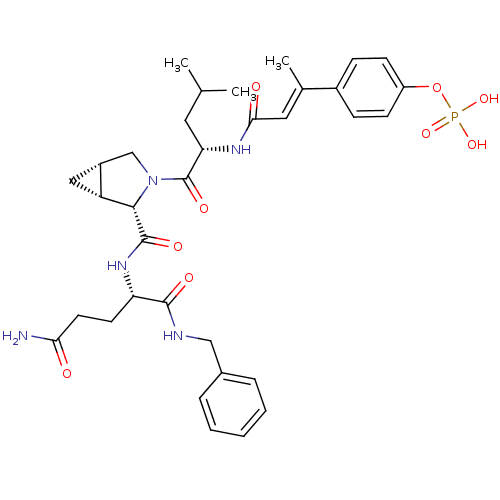
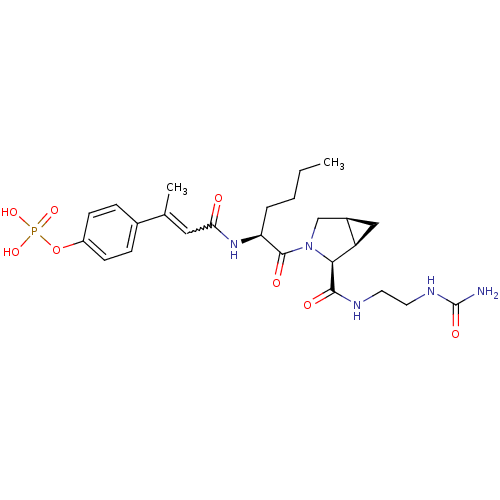
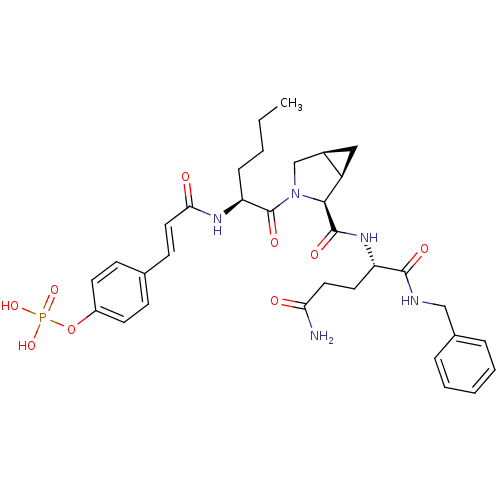
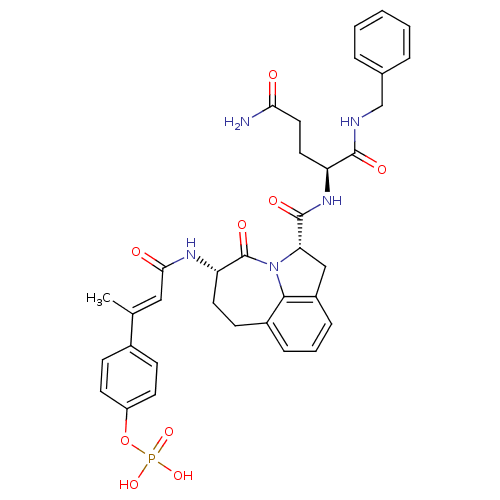
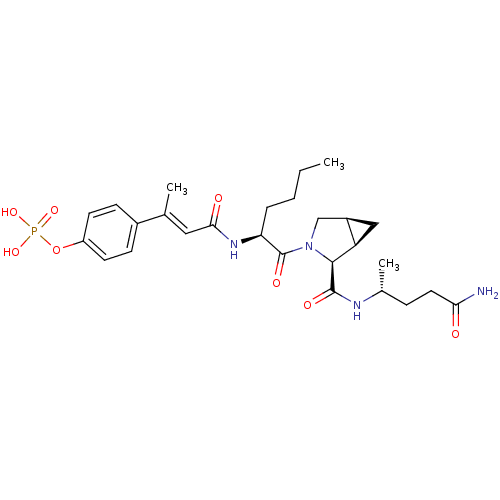
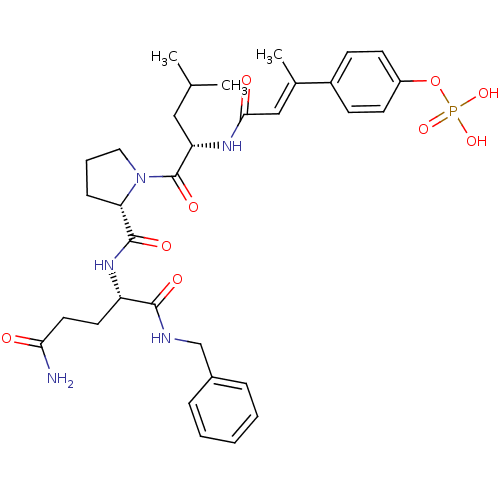
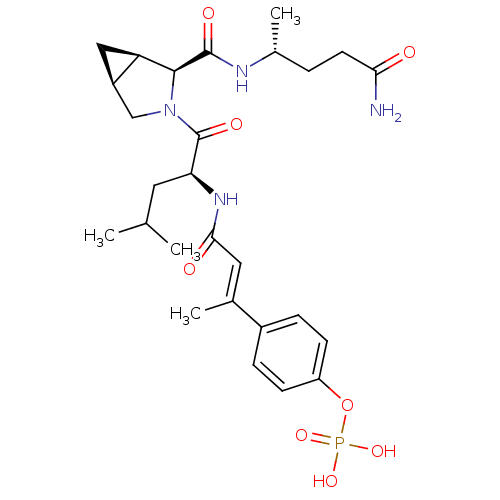
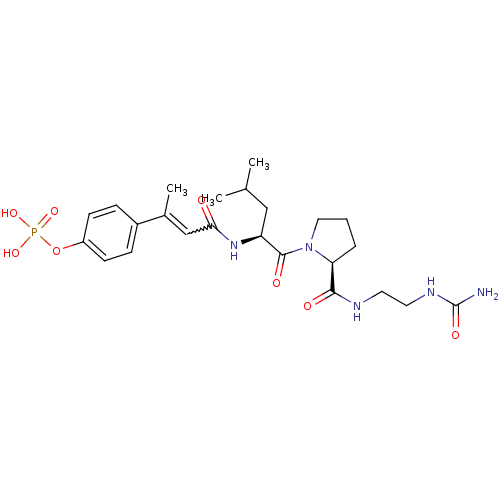
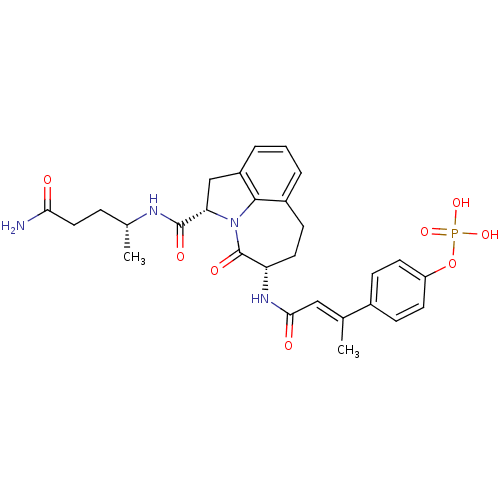
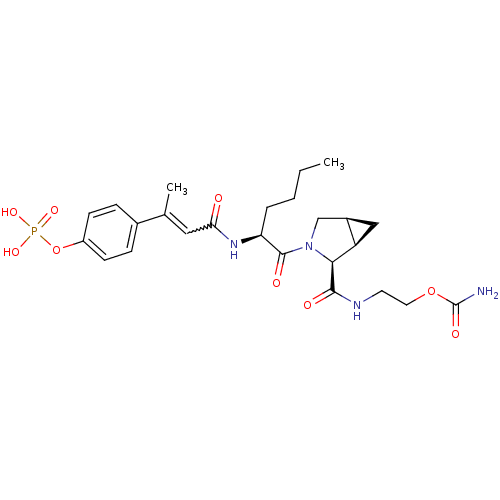
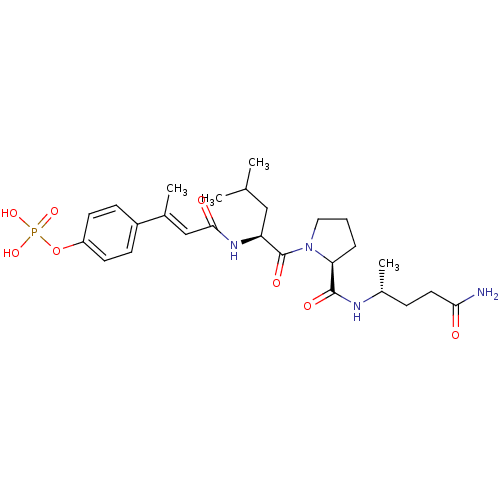
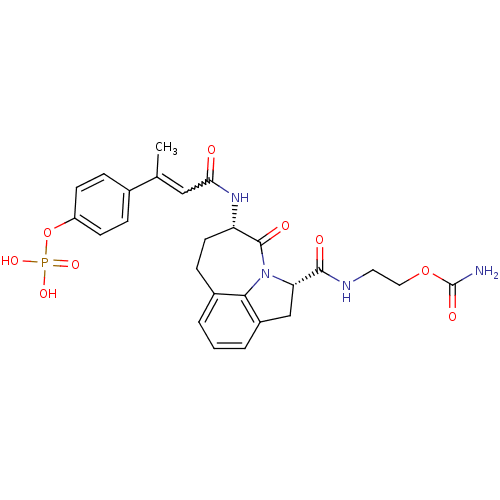
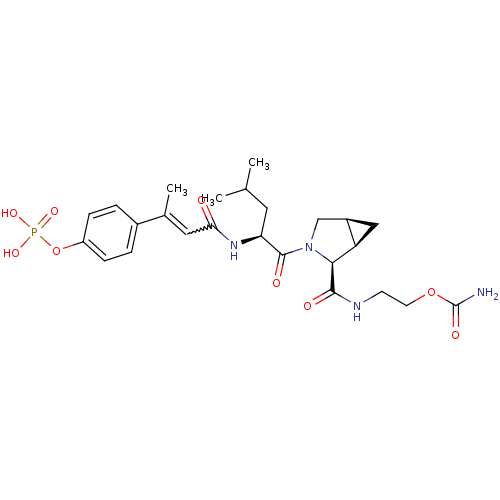
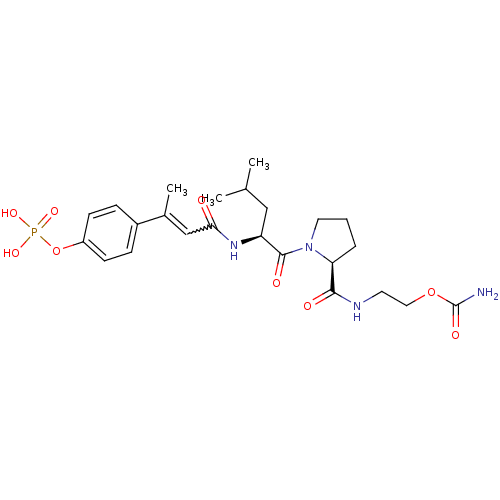
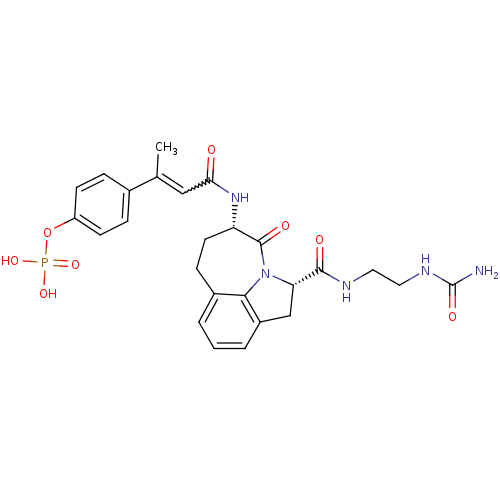

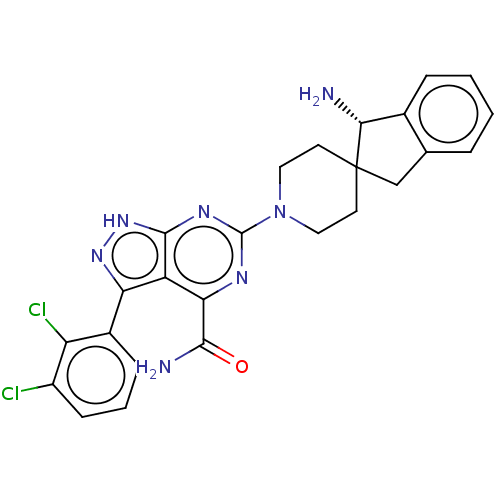

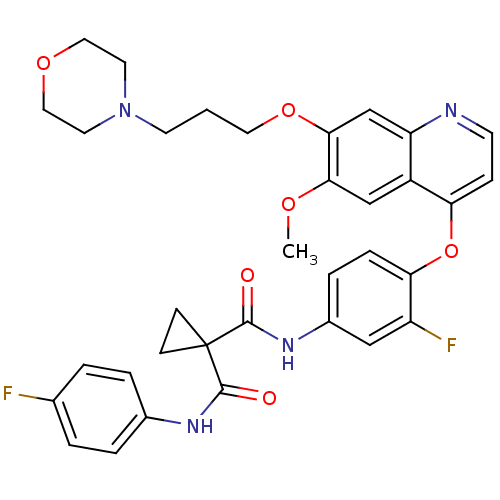
 3D Structure (crystal)
3D Structure (crystal)