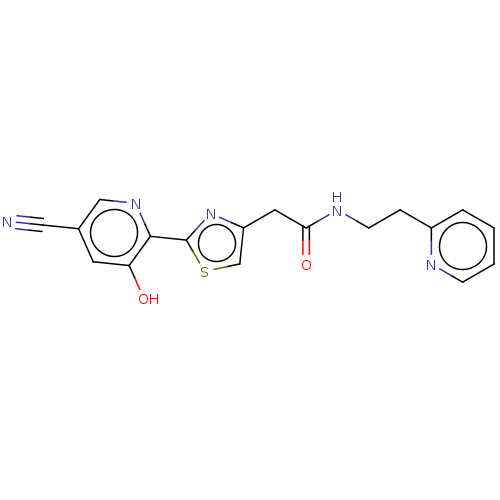
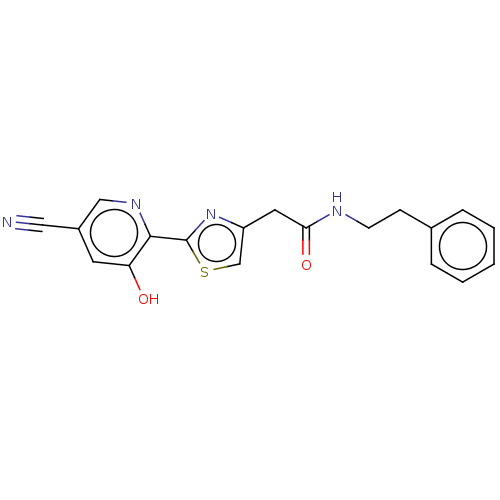
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0700nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.100nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60nMAssay Description:In order to evaluate the activity of the compounds of the present invention as a BTK inhibitor, commercially available BTK (Promega) was used for thi...More data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of PHD2 (unknown origin) after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of PHD2 (unknown origin) after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of PHD2 (unknown origin) after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.60E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.40E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+3nMAssay Description:Inhibition of PHD2 in human Hep3B cells assessed as erythropoietin secretion by ELISAMore data for this Ligand-Target Pair

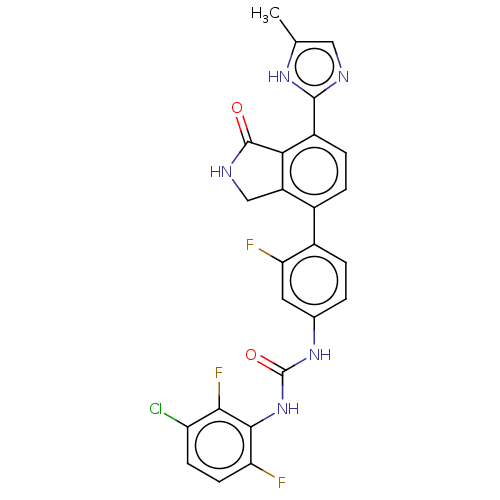
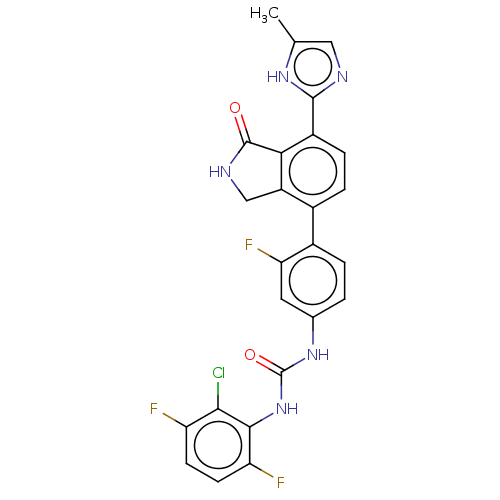
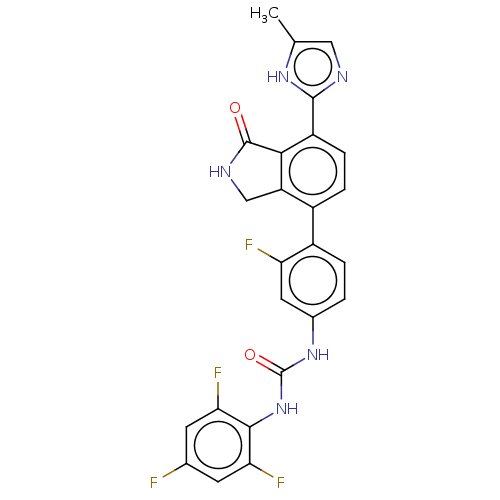
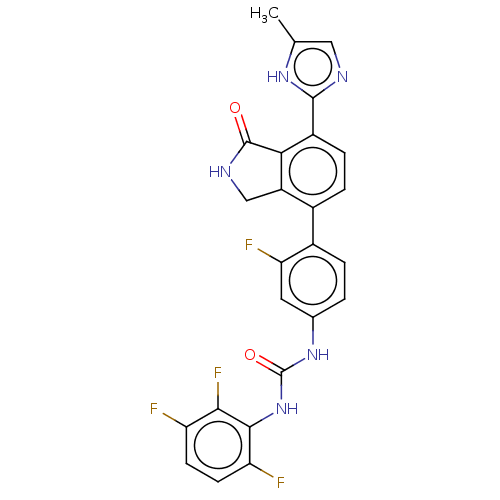
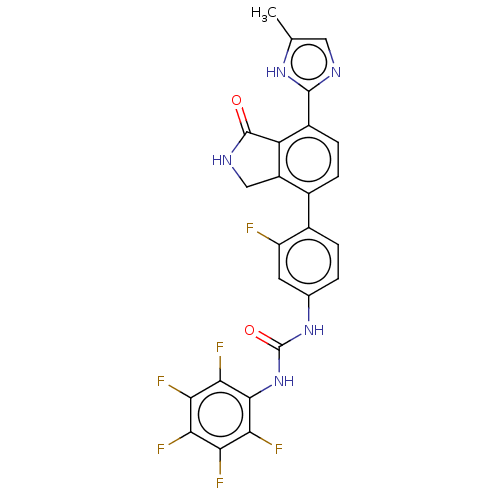
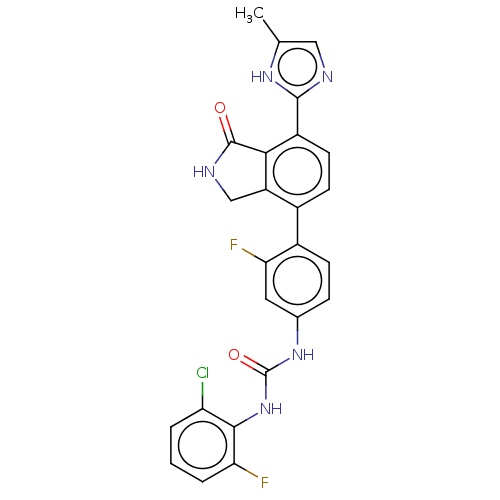
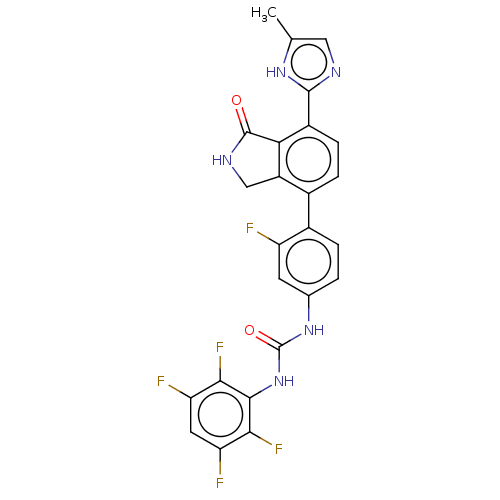
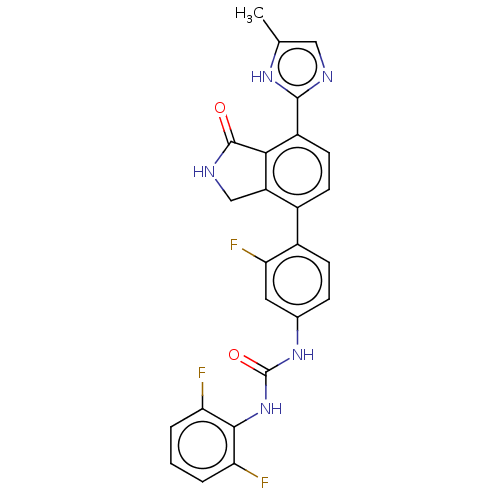
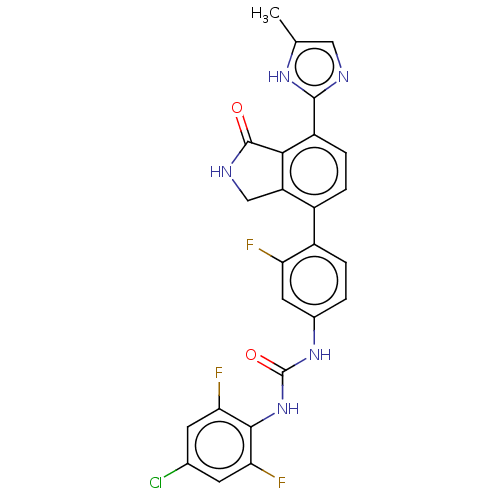
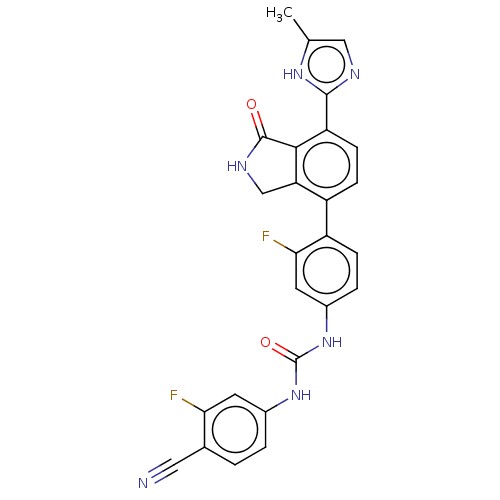
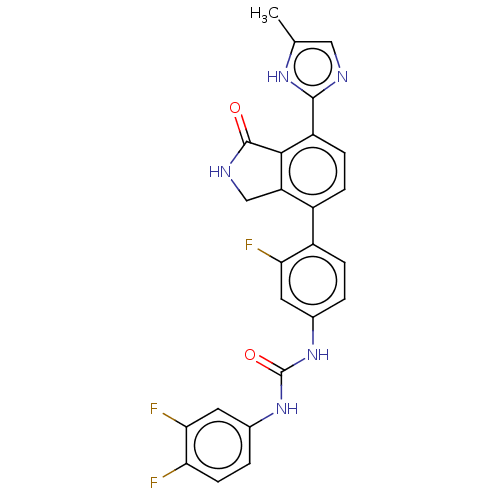
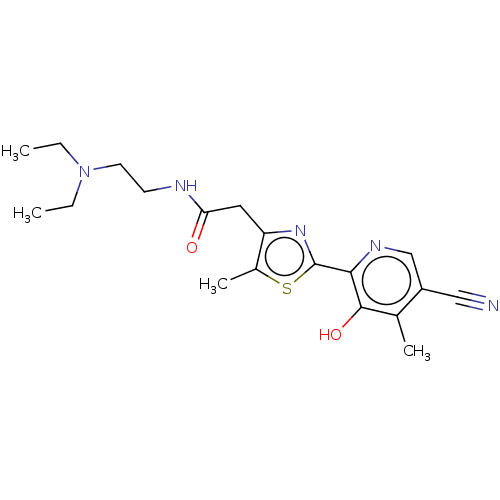

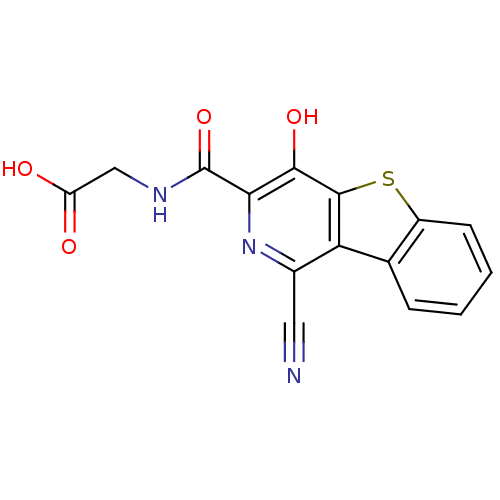
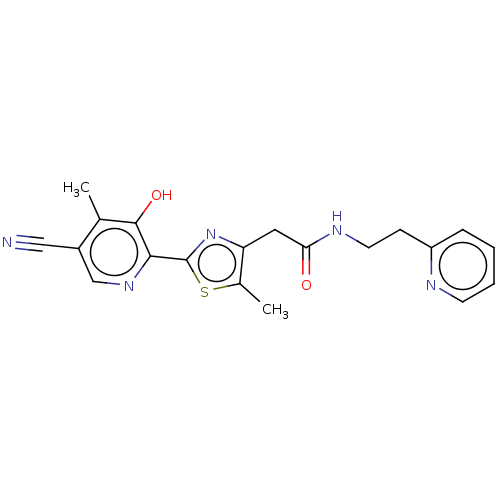
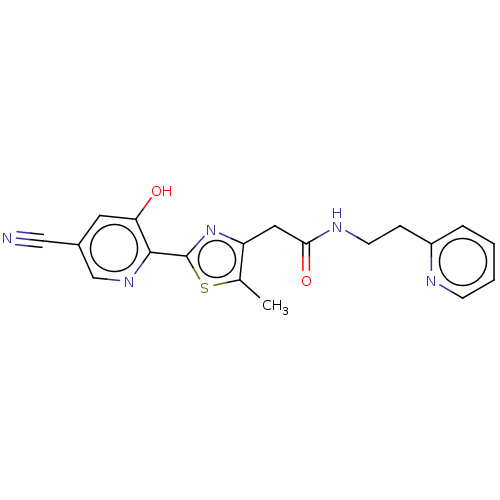
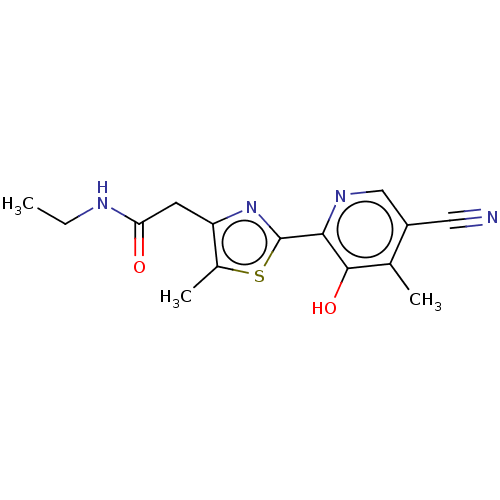
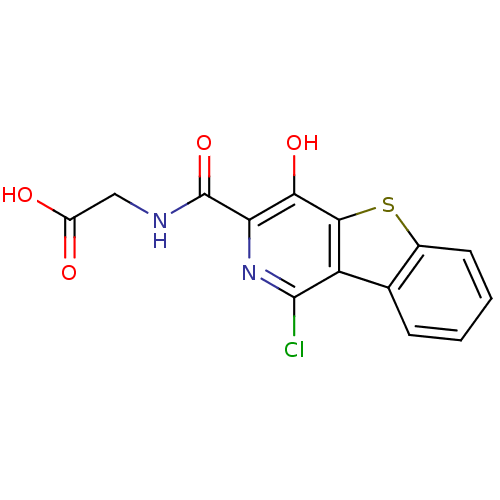
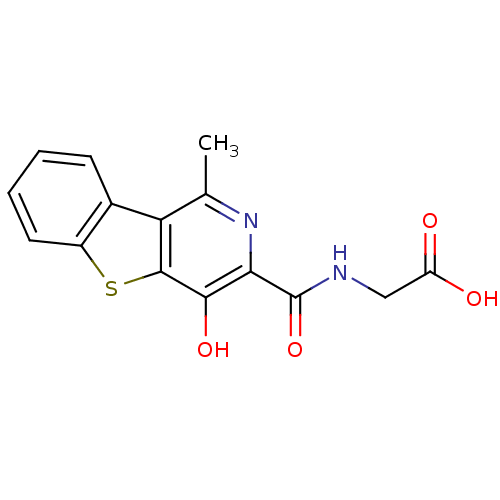
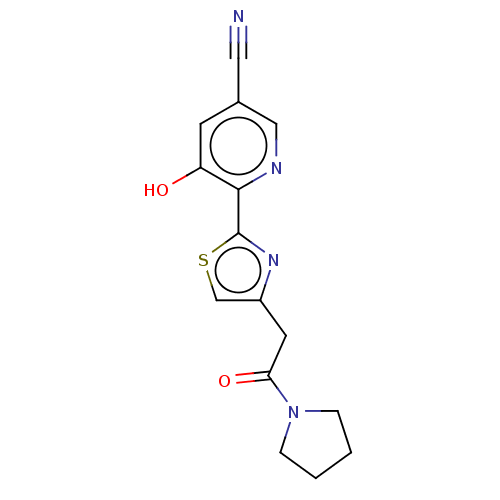
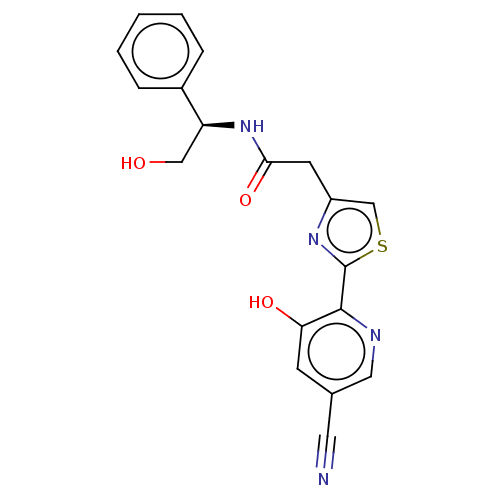
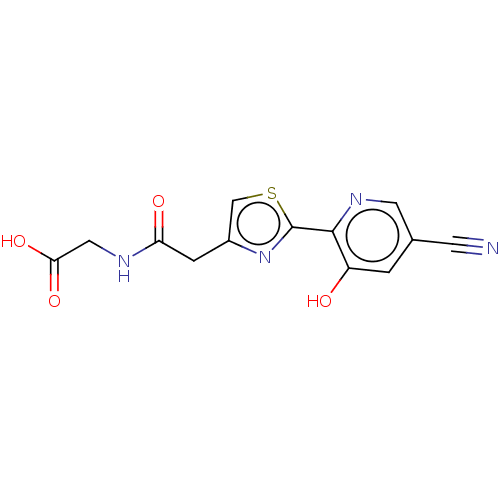

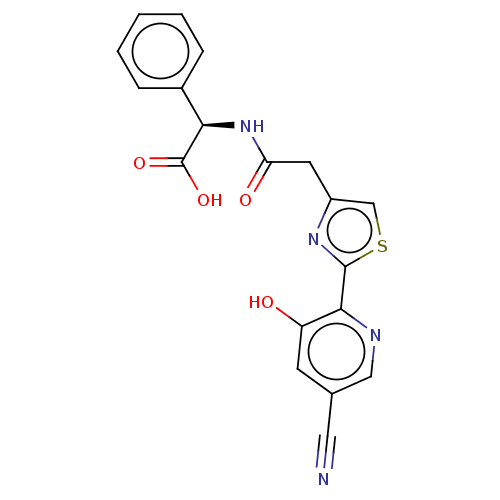
 3D Structure (crystal)
3D Structure (crystal)