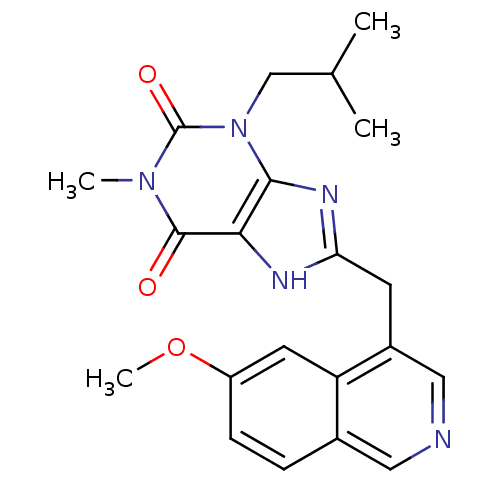
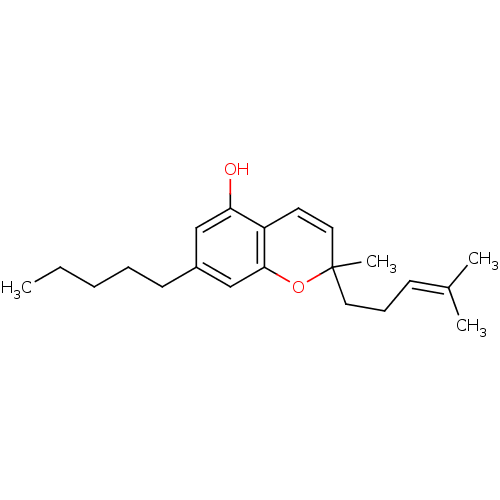
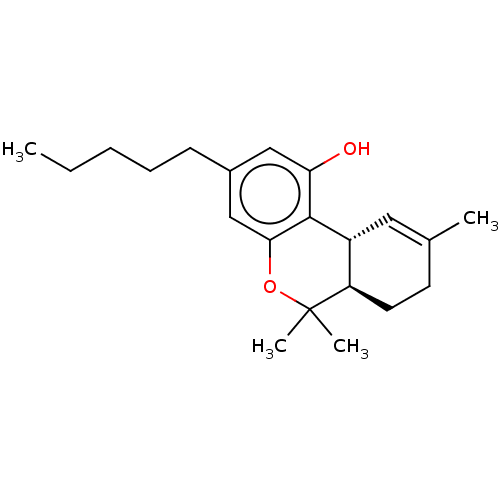
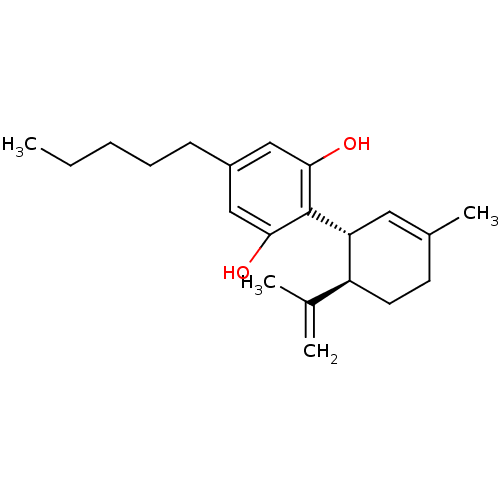
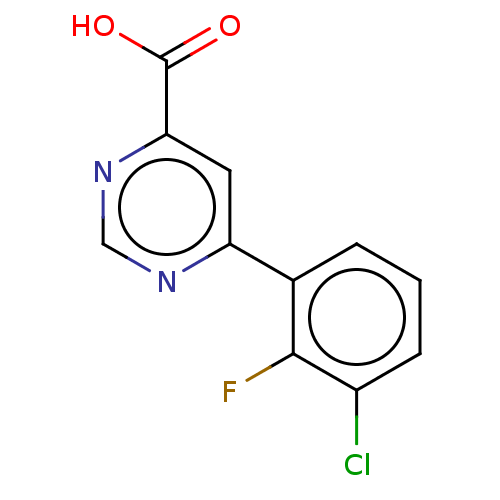
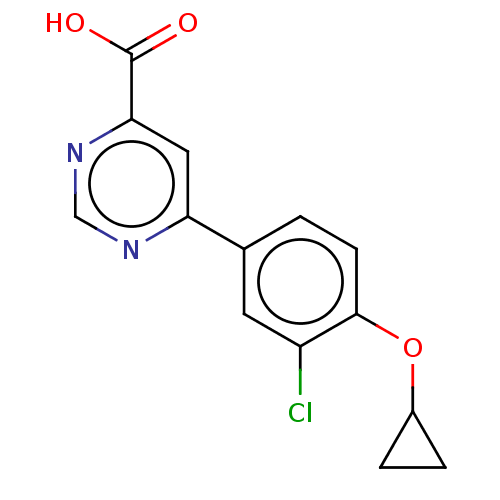
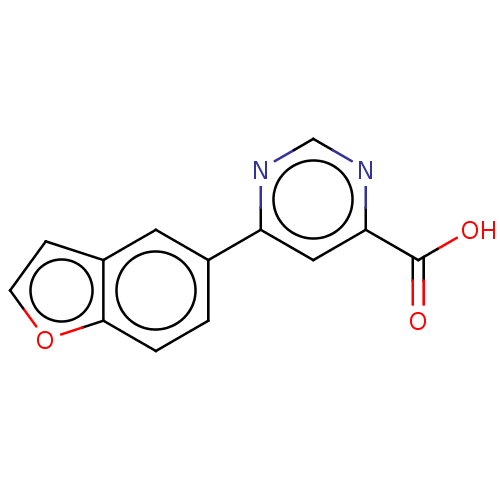
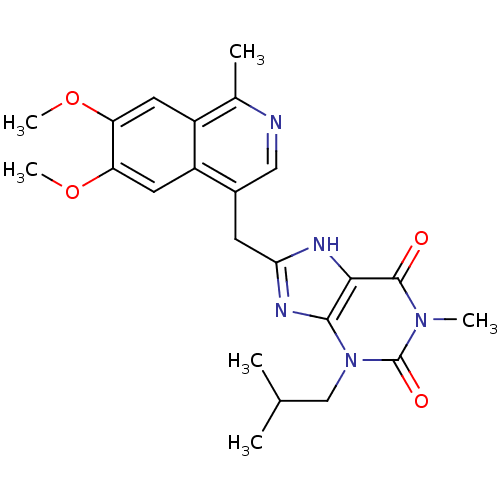
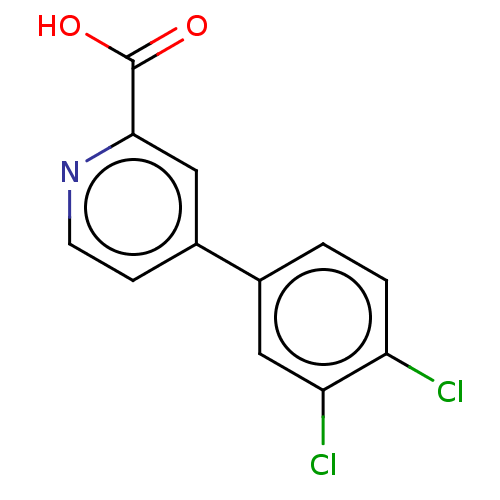
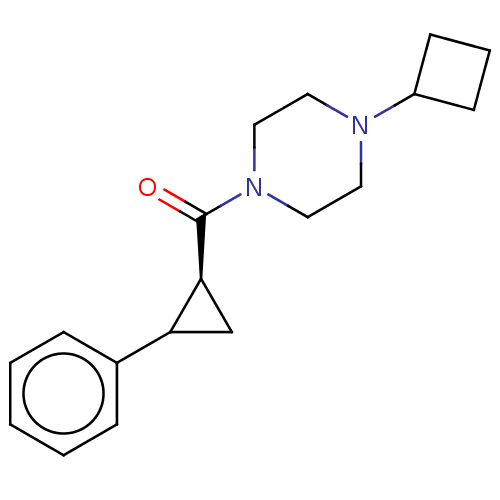
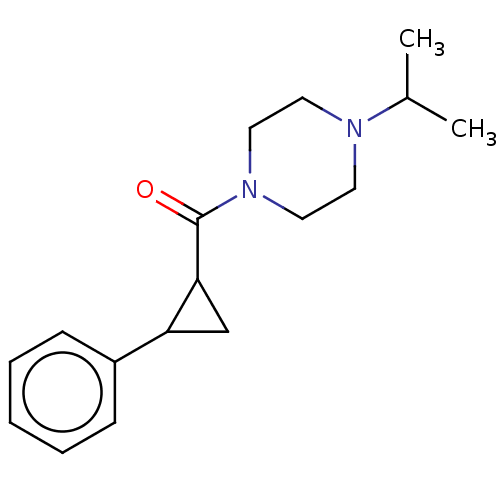
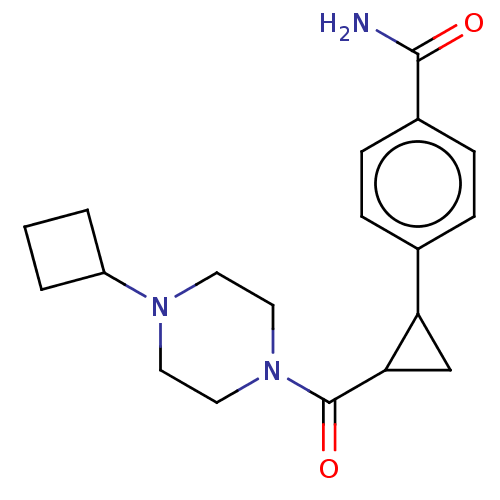
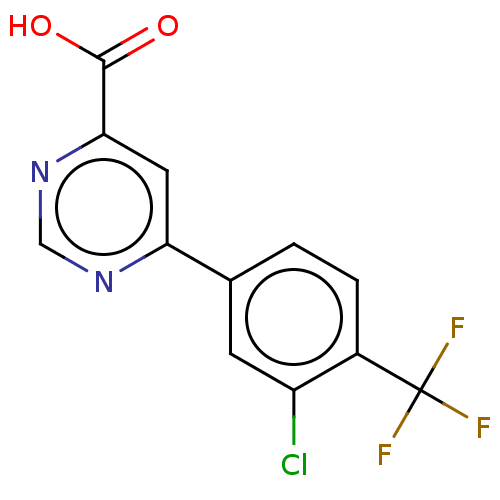
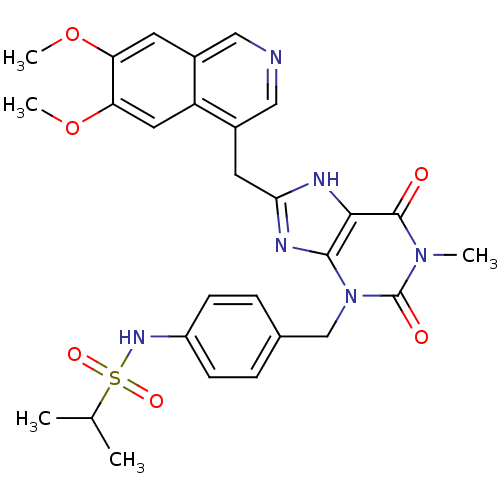
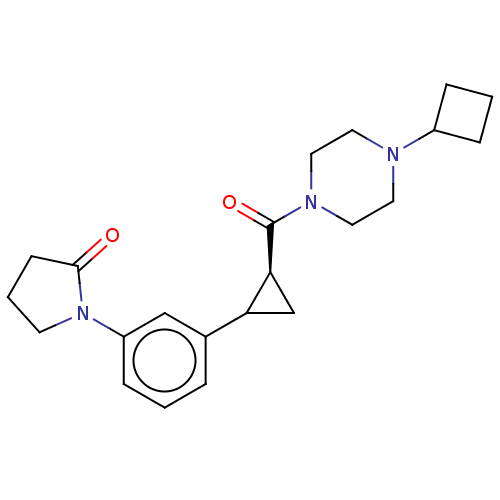
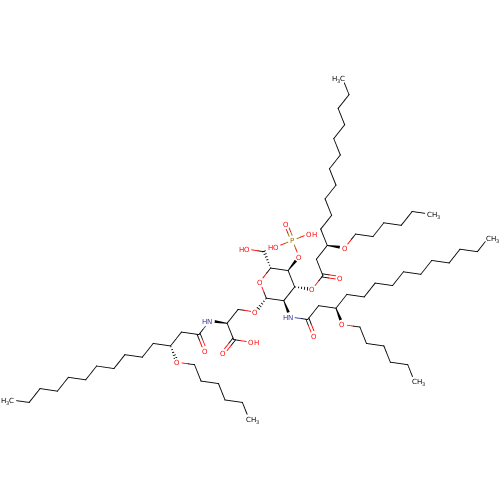
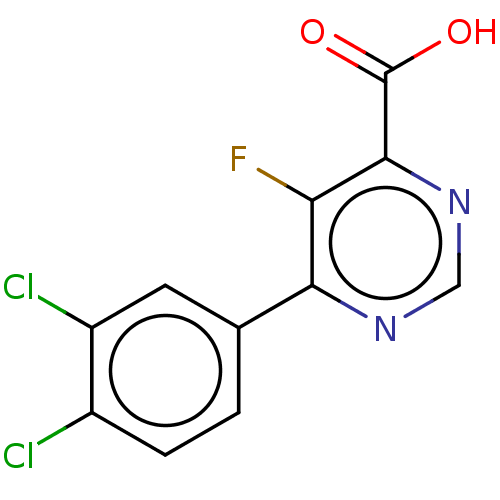
TargetAdenosine receptor A1(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of adenosine A1 receptorMore data for this Ligand-Target Pair
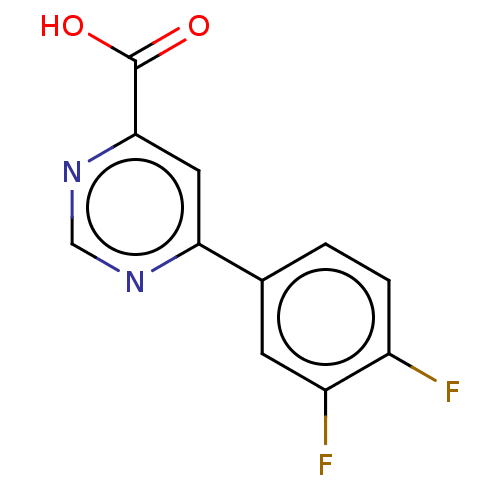
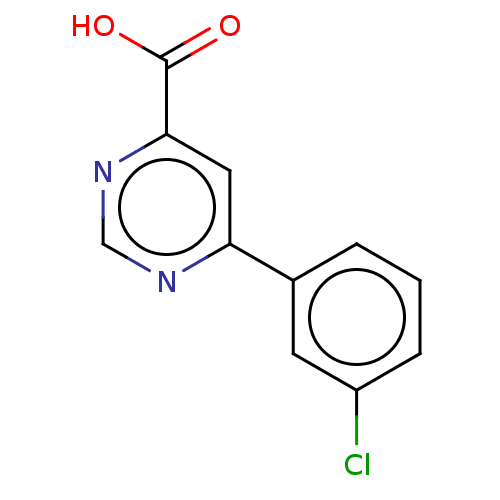
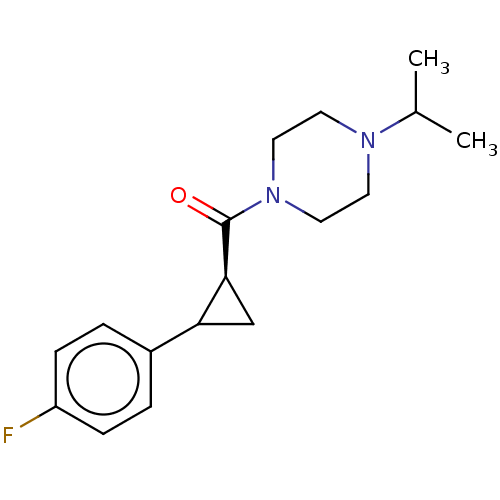
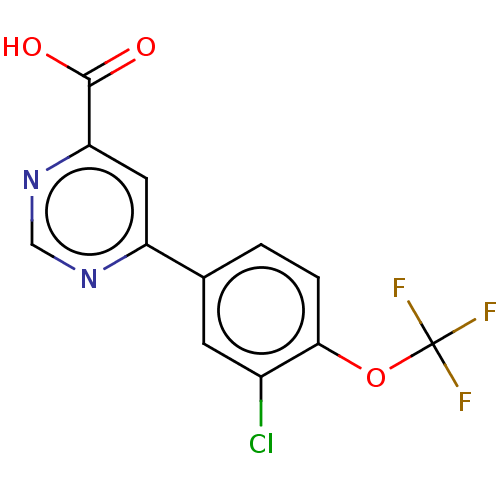
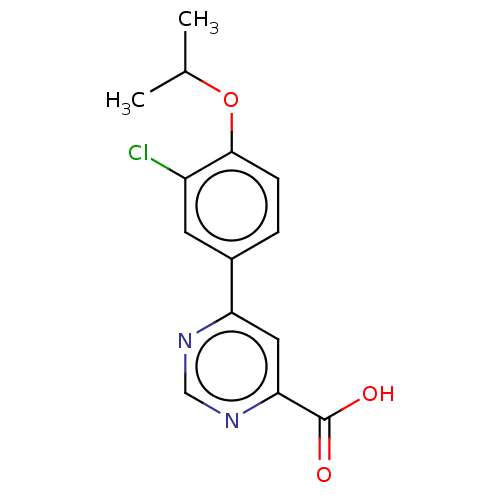
TargetL-lactate dehydrogenase A chain(Homo sapiens (Human))
The University Of Sydney
Curated by ChEMBL
The University Of Sydney
Curated by ChEMBL
Affinity DataKi: 6.50E+3nMAssay Description:Non-competitive inhibition of human LDHA assessed as reduction in lactate production using pyruvate as substrate in presence of NADH by Lineweaver-Bu...More data for this Ligand-Target Pair
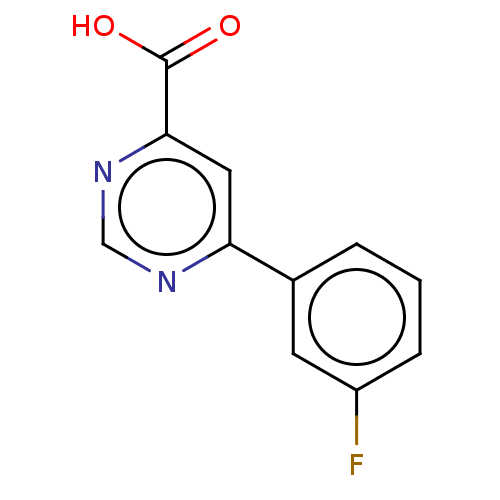
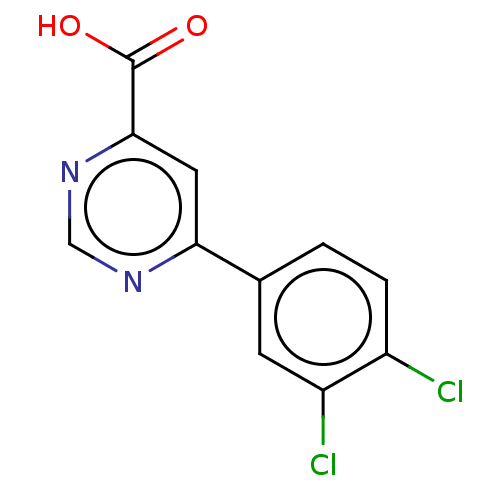
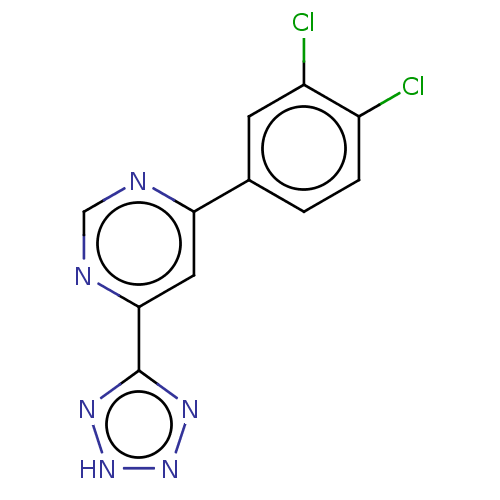
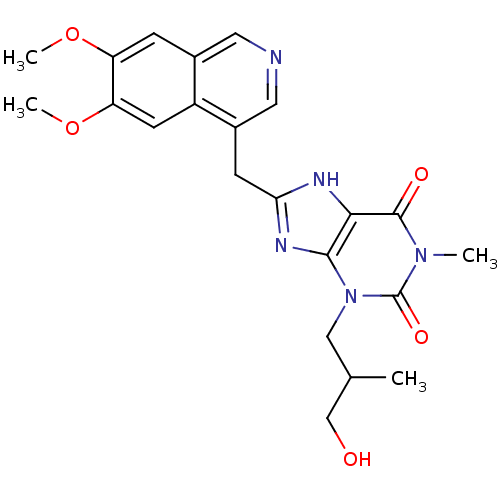
TargetL-lactate dehydrogenase A chain(Homo sapiens (Human))
The University Of Sydney
Curated by ChEMBL
The University Of Sydney
Curated by ChEMBL
Affinity DataKi: 8.50E+3nMAssay Description:Non-competitive inhibition of human LDHA assessed as reduction in lactate production using pyruvate as substrate in presence of NADH by Lineweaver-Bu...More data for this Ligand-Target Pair
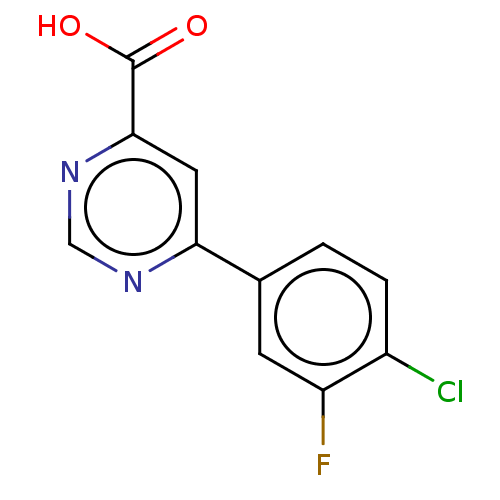
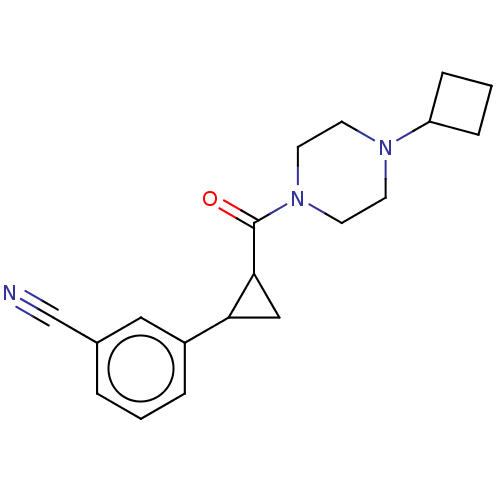
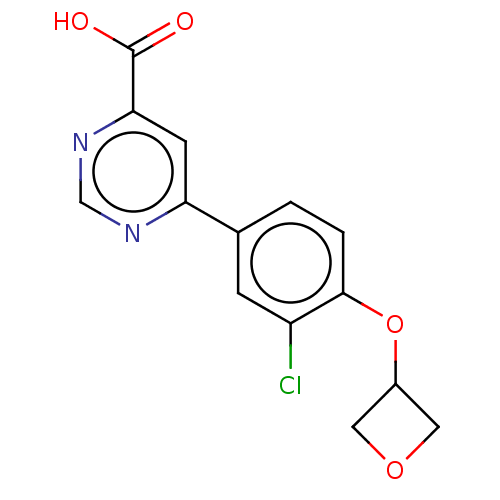
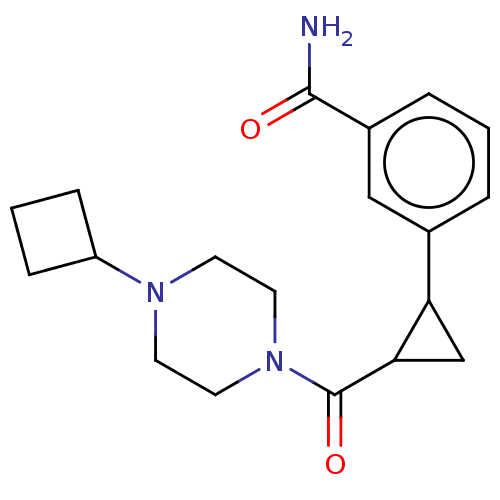
TargetL-lactate dehydrogenase A chain(Homo sapiens (Human))
The University Of Sydney
Curated by ChEMBL
The University Of Sydney
Curated by ChEMBL
Affinity DataKi: 1.40E+4nMAssay Description:Non-competitive inhibition of human LDHA assessed as reduction in lactate production using pyruvate as substrate in presence of NADH by Lineweaver-Bu...More data for this Ligand-Target Pair
TargetL-lactate dehydrogenase A chain(Homo sapiens (Human))
The University Of Sydney
Curated by ChEMBL
The University Of Sydney
Curated by ChEMBL
Affinity DataKi: 2.40E+4nMAssay Description:Non-competitive inhibition of human LDHA assessed as reduction in lactate production using pyruvate as substrate in presence of NADH by Lineweaver-Bu...More data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.700nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.834nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.37nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.5nMAssay Description:Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.63nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
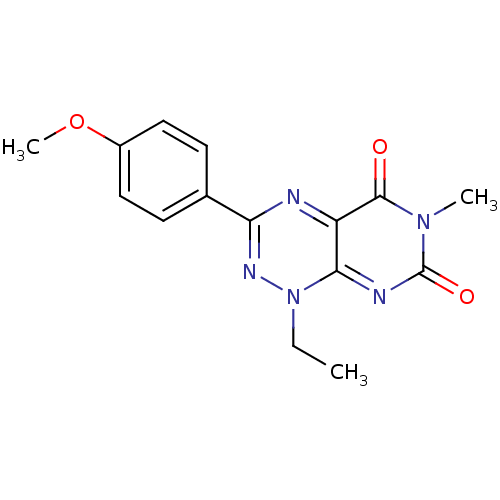
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Antagonist activity at TLR4 in human PBMC assessed as inhibition of LPS-stimulated TNFalpha production preincubated for 30 mins before LPS challenge ...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.41nMpH: 7.4 T: 2°CAssay Description:A GTP gamma S binding assay can be used to investigate antagonist properties of compounds in CHO cells (Chinese Hamster Ovary) transfected with human...More data for this Ligand-Target Pair
Affinity DataIC50: 3.49nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 3.69nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 3.81nMpH: 7.4 T: 2°CAssay Description:A GTP gamma S binding assay can be used to investigate antagonist properties of compounds in CHO cells (Chinese Hamster Ovary) transfected with human...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10nMAssay Description:Inhibition of rat KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.09nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair
Affinity DataIC50: 5.10nMAssay Description:Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30nMAssay Description:Antagonist activity at TLR4 in human PBMC assessed as inhibition of LPS-stimulated TNFalpha production preincubated for 30 mins before LPS challenge ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.83nMpH: 7.4 T: 2°CAssay Description:A GTP gamma S binding assay can be used to investigate antagonist properties of compounds in CHO cells (Chinese Hamster Ovary) transfected with human...More data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of human KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
TargetcGMP-specific 3',5'-cyclic phosphodiesterase(Homo sapiens (Human))
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Novartis Institutes Of Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of human platelet PDE5 by [3H]cGMP scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.19nMpH: 7.4 T: 2°CAssay Description:A GTP gamma S binding assay can be used to investigate antagonist properties of compounds in CHO cells (Chinese Hamster Ovary) transfected with human...More data for this Ligand-Target Pair
Affinity DataIC50: 6.20nMAssay Description:Inhibition of mouse KMO assessed as conversion of kynurenine to 3-hydroxykynurenine by LC-MS/MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.40nMAssay Description:The H3 binding assay was/can be used to evaluate the ability of at least one compound in accordance with formula I, Ia, Ib, and/or Ic to inhibit [3H]...More data for this Ligand-Target Pair