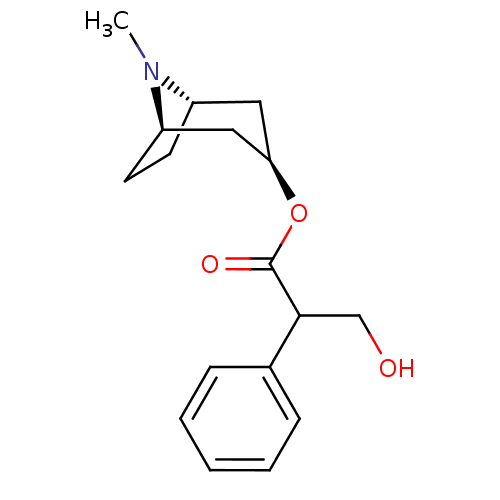
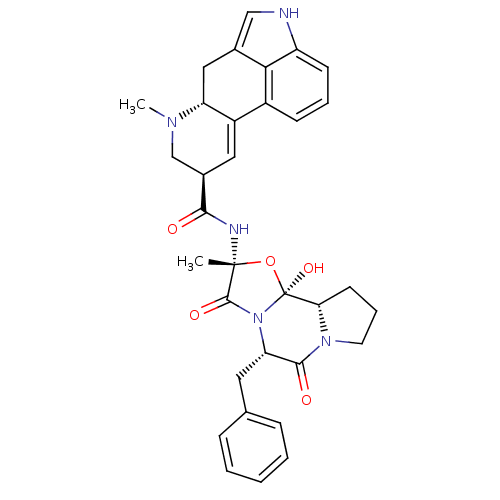
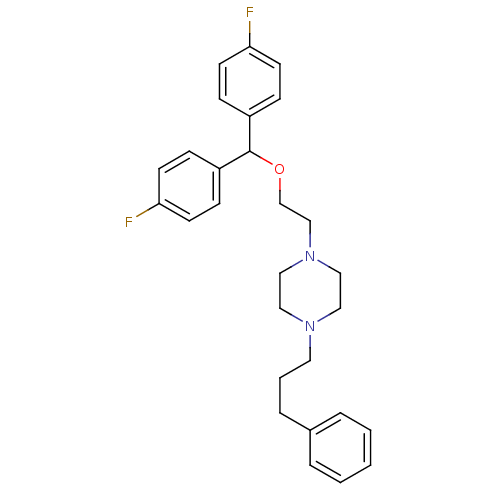
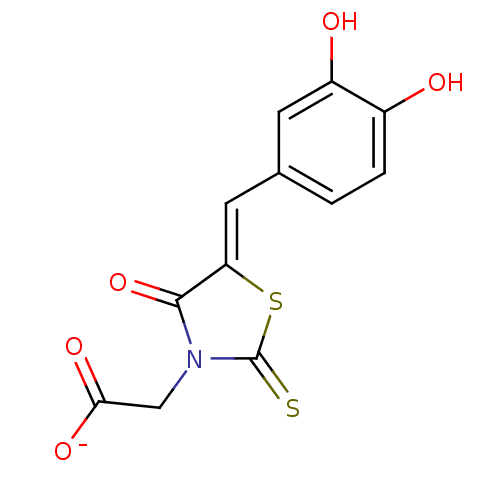
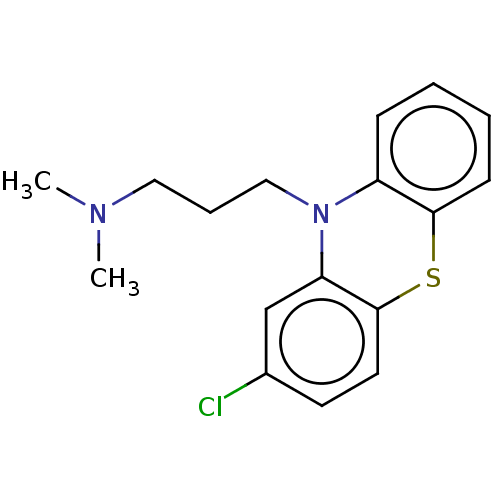
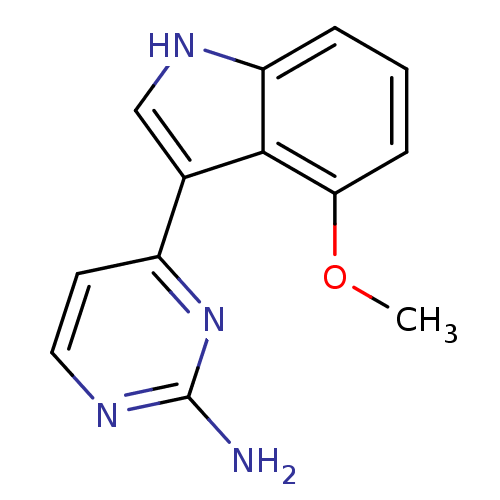
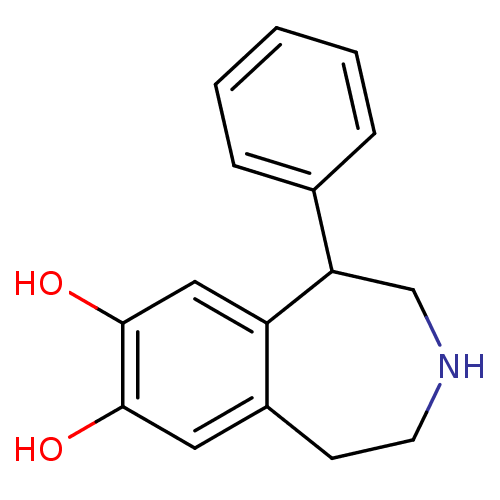
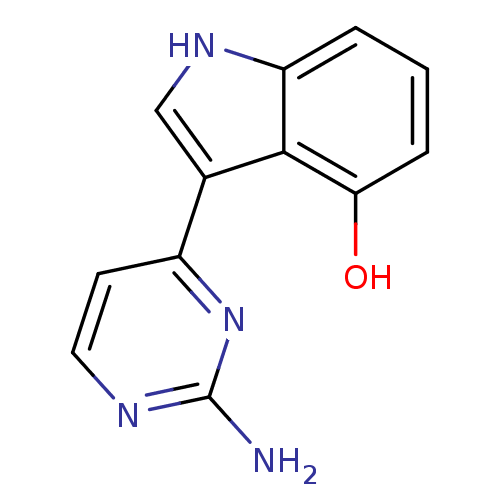
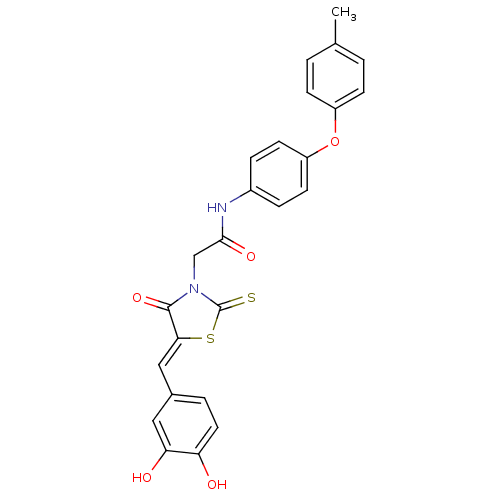
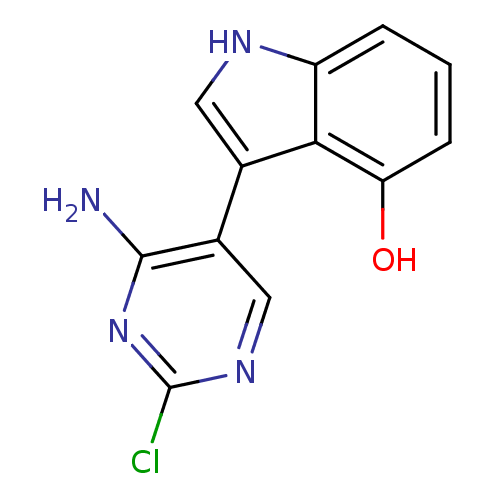
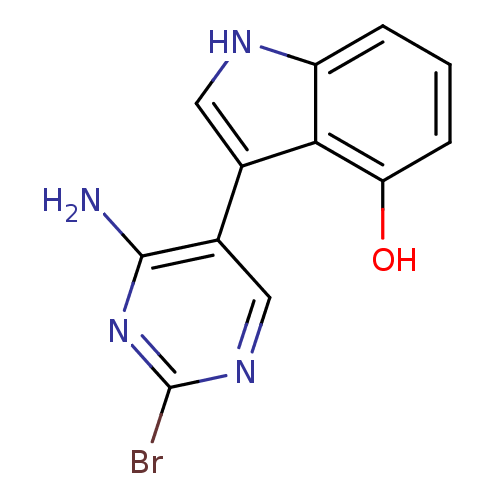
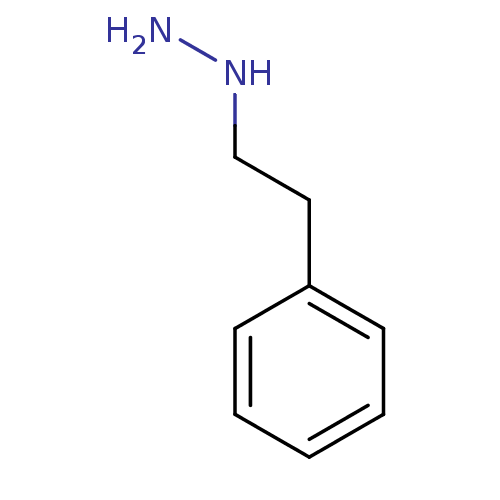
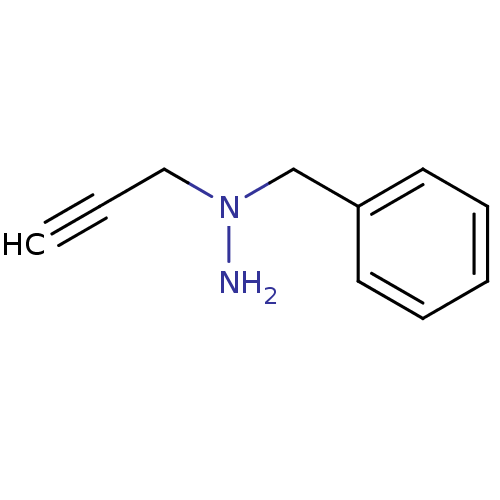
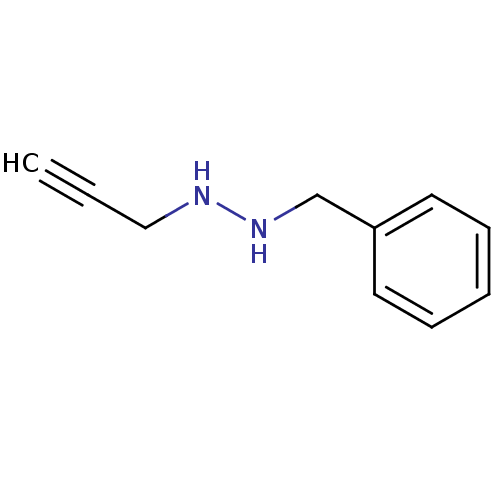
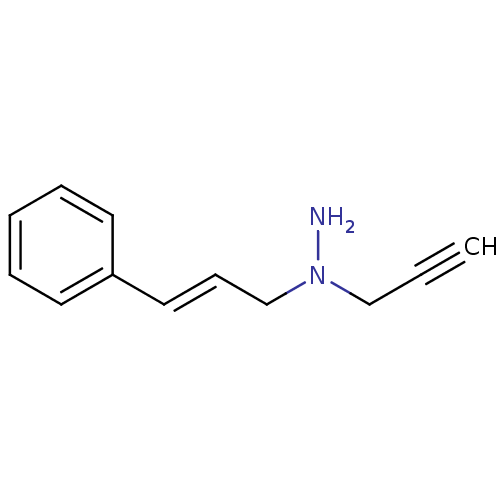
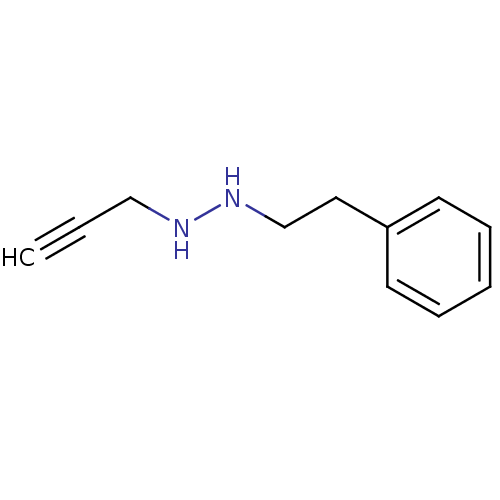
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 6nMAssay Description:Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 5A(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 14nMAssay Description:Displacement of [3H]LSD from 5-HT5A receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 22nMAssay Description:Displacement of [3H]WIN35428 from DAT after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 26nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Displacement of [3H]LSD from 5-HT7 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 27nMAssay Description:Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 42nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 88nMAssay Description:Displacement of [3H]LSD from 5-HT2B receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 100nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 124nMAssay Description:Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2B(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 150nMAssay Description:Displacement of [3H]LSD from 5-HT2B receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 202nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 620nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 684nMAssay Description:Displacement of [3H]8-OH-DPAT from 5-HT1A receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 1.35E+3nMAssay Description:Displacement of [3H]8-OH-DPAT from 5-HT1A receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 5A(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 1.53E+3nMAssay Description:Displacement of [3H]LSD from 5-HT5A receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 7(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 1.99E+3nMAssay Description:Displacement of [3H]LSD from 5-HT7 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 2.35E+3nMAssay Description:Displacement of [3H]WIN35428 from DAT after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: 7.04E+3nMAssay Description:Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 7.90E+3nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]QNB from muscarinic acetylcholine M5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]SCH233930 from dopamine D5 receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1D(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]5-carboximidotryptamine from 5-HT1D receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataKi: >1.00E+4nMAssay Description:Displacement of [3H]mesulergine from 5-HT2C receptor after 1.5 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >2.50E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMpH: 7.4Assay Description:All reactions were monitored spectrophotometrically at 340 nm by using initial rates from the first 5% of reaction.More data for this Ligand-Target Pair
Affinity DataIC50: 2.80nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 76nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 77nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 121nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 362nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 516nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+3nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase B activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 1.17E+3nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
TargetGlycogen synthase kinase-3 beta(Homo sapiens (Human))
University Of South Florida
Curated by ChEMBL
University Of South Florida
Curated by ChEMBL
Affinity DataIC50: 1.30E+3nMAssay Description:Inhibition of GSK3-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 1.56E+3nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 1.85E+3nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair
Affinity DataIC50: 2.04E+3nMAssay Description:In vitro ability of the compound to inhibit Monoamine oxidase A activity in rat whole brain in vitroMore data for this Ligand-Target Pair