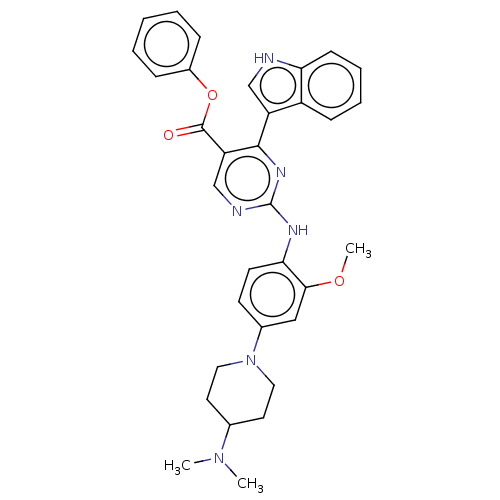
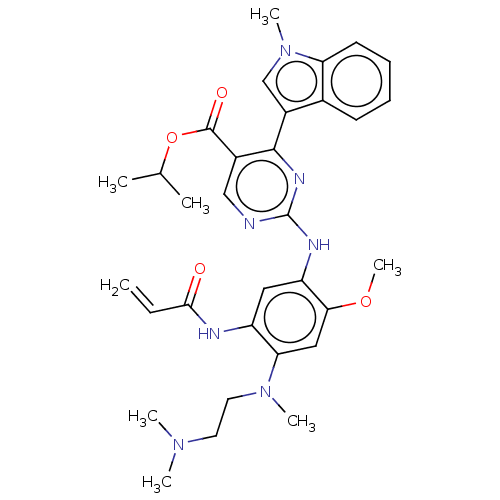
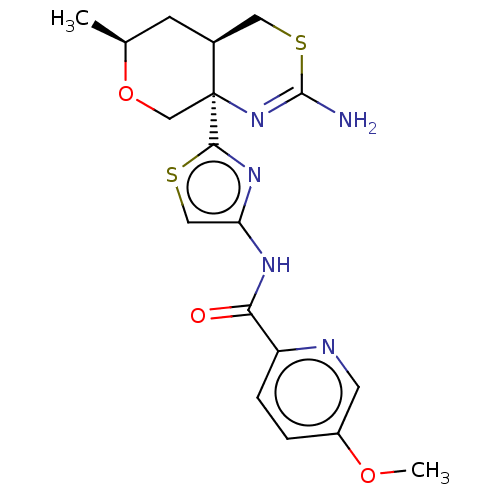
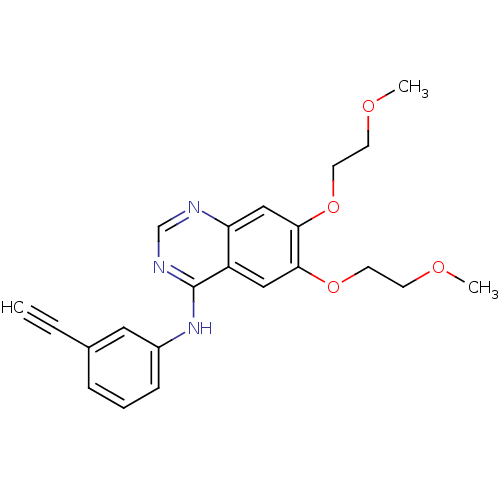
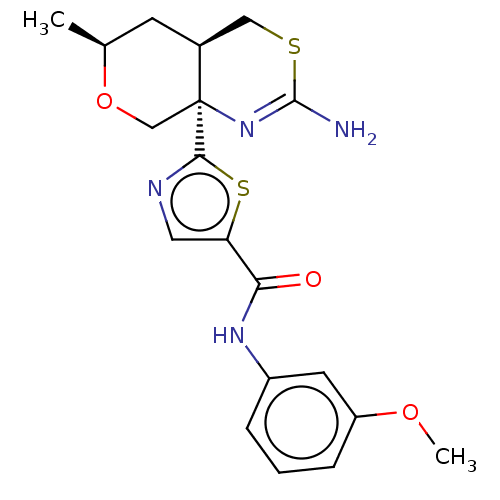
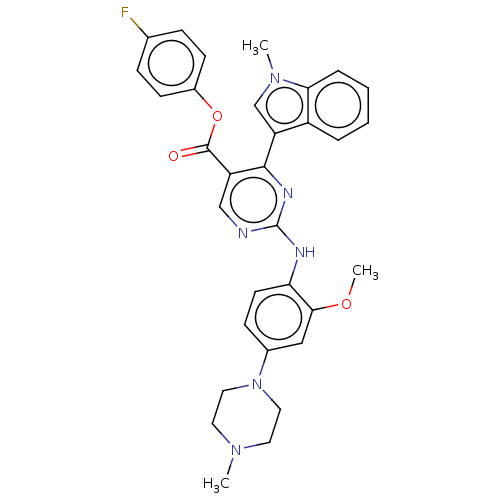
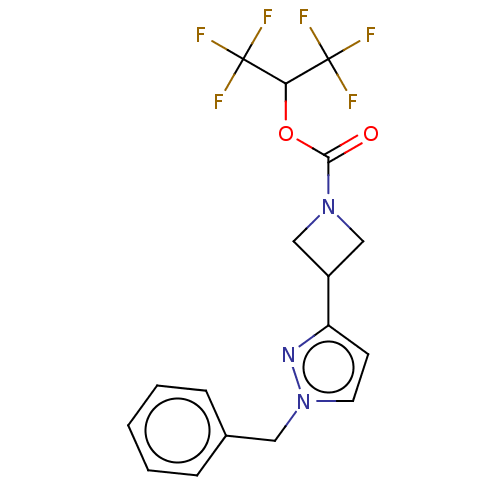
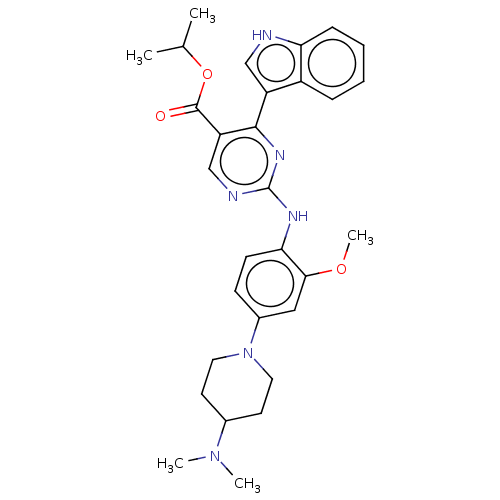
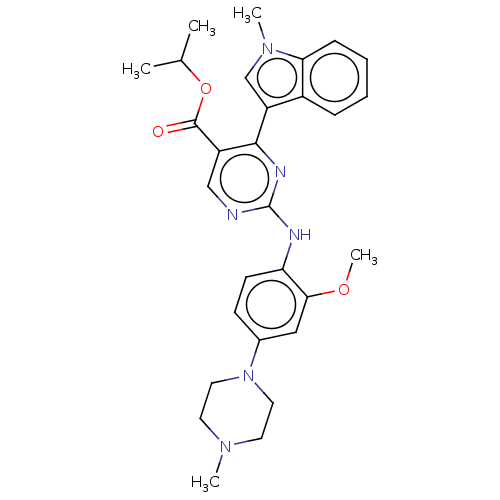
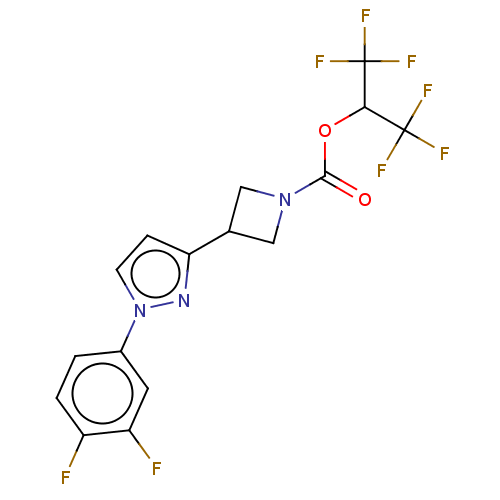
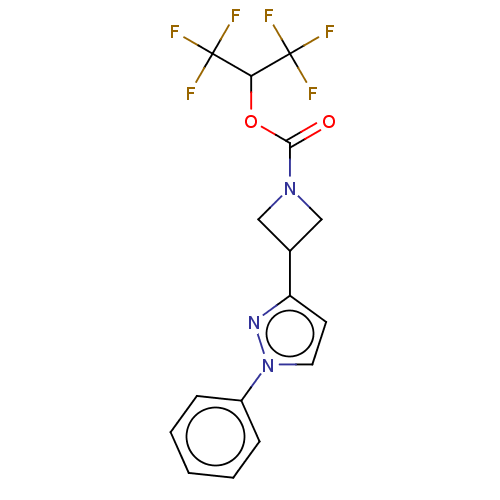
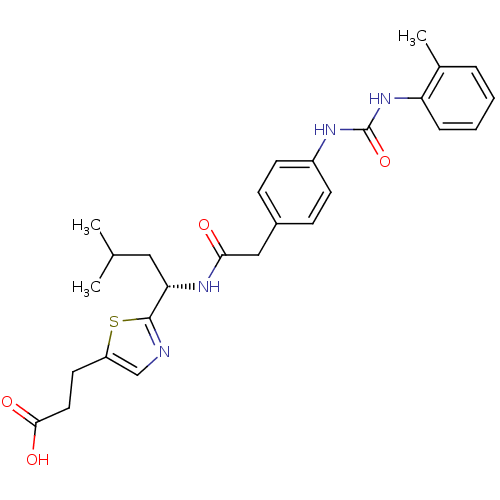
TargetNeuraminidase(Influenza B virus (B/Lee/40))
University Of Alabama At Birmingham
Curated by ChEMBL
University Of Alabama At Birmingham
Curated by ChEMBL
Affinity DataKi: 2.5nMAssay Description:In vitro inhibitory activity of the compound against B/Lee/40 Influenza B Neuraminidase.More data for this Ligand-Target Pair
Affinity DataKi: 8.40nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 13.3nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 15.5nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 18nMAssay Description:Inhibition of cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 18.3nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibition of cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 27nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 62nMAssay Description:Inhibition of cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 83nMAssay Description:Inhibition of cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 99nMAssay Description:Inhibition of cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assayMore data for this Ligand-Target Pair
Affinity DataKi: 600nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: 1.08E+3nMAssay Description:Inhibitory activity against human Calpain 1 isolated from erythrocytesMore data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+4nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Binding affinity against HIV reverse transcriptase (Estimated Ki)More data for this Ligand-Target Pair
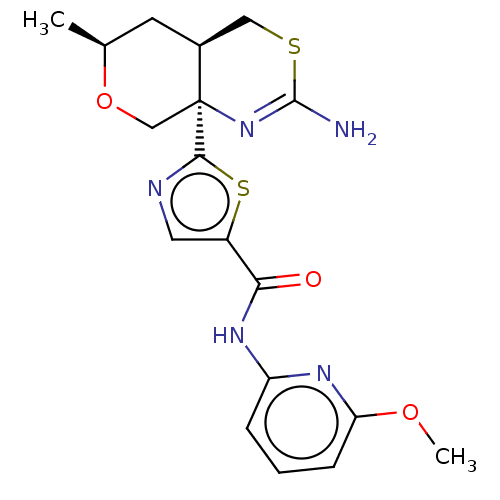
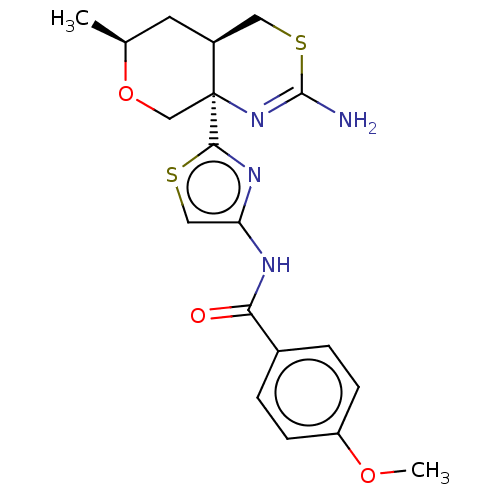
Affinity DataIC50: 0.00600nMAssay Description:Inhibition of BACE1 (unknown origin) using biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization ass...More data for this Ligand-Target Pair
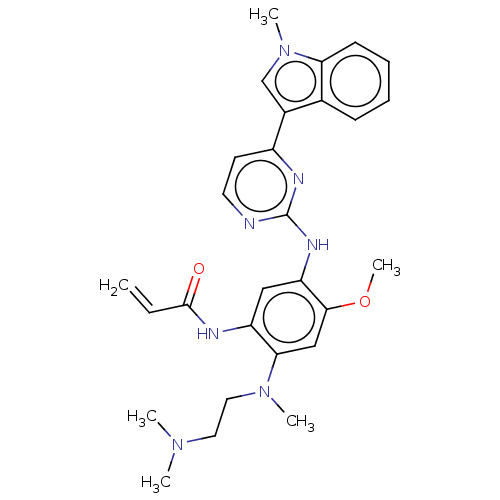
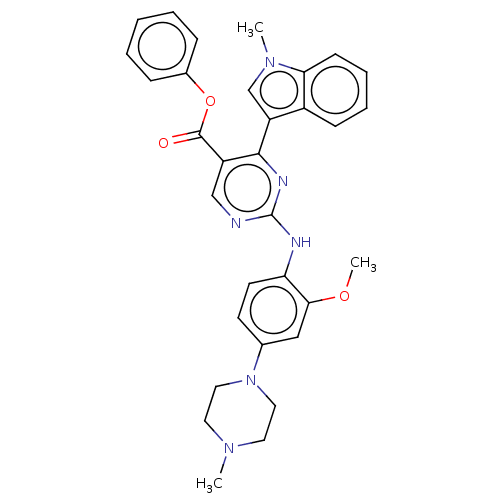
Affinity DataIC50: 0.00800nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
Ligand InfoPDB
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 1...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0140nMAssay Description:Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0200nMAssay Description:Inhibition of EGFR L858R/C797S mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition...More data for this Ligand-Target Pair
Ligand InfoPDB
Affinity DataIC50: 0.0240nMAssay Description:Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0250nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0290nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0330nMAssay Description:Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0340nMAssay Description:Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 1...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0530nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
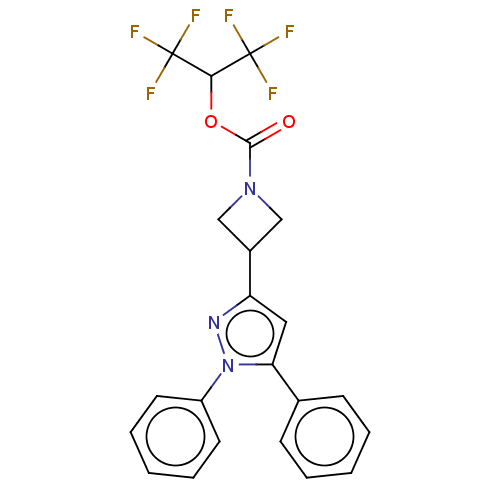
Ligand Info
Affinity DataIC50: 0.0590nMAssay Description:Inhibition of EGFR L858R/C797S mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0640nMAssay Description:Inhibition of EGFR L858R/C797S mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition...More data for this Ligand-Target Pair
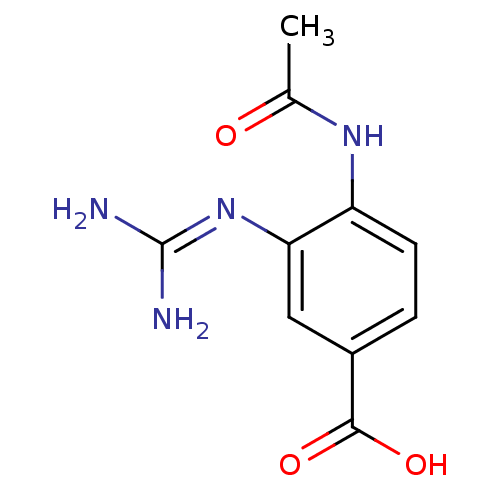
Ligand Info
Affinity DataIC50: 0.110nMAssay Description:Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for...More data for this Ligand-Target Pair
Affinity DataIC50: 0.150nMAssay Description:Inhibition of EGFR L858R/C797S mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition...More data for this Ligand-Target Pair
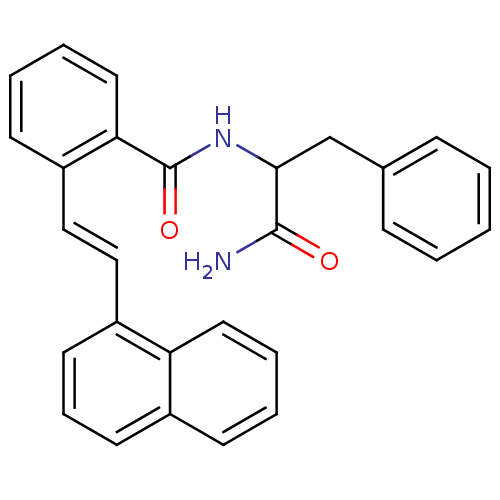
Ligand Info
Affinity DataIC50: 0.160nMAssay Description:Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
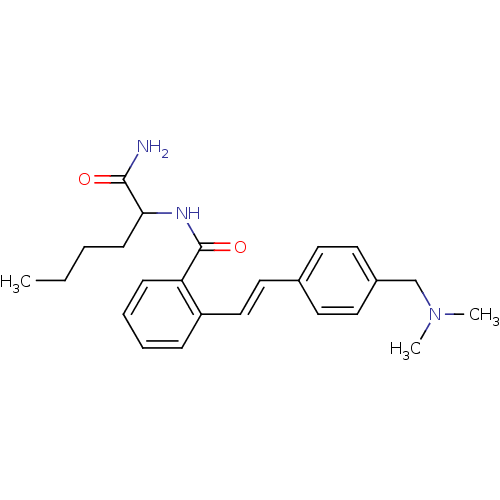
Ligand Info
Affinity DataIC50: 0.170nMAssay Description:Inhibition of EGFR L858R mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 1...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.180nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.240nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.25nMAssay Description:Inhibition of human recombinant sVCAM-1 binding to alpha4-beta1 integrin (VLA-4) in ELISAMore data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Inhibition of wild type EGFR (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition for 25 m...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant MAGL using 7-HCA as substrate preincubated for 30 mins followed by substrate addition measured after 60 mins by fluor...More data for this Ligand-Target Pair
Affinity DataIC50: 0.320nMAssay Description:Inhibition of EGFR L858R/C797S mutant (unknown origin) using TK-substrate preincubated with enzyme for 30 mins followed by substrate and ATP addition...More data for this Ligand-Target Pair
Ligand Info

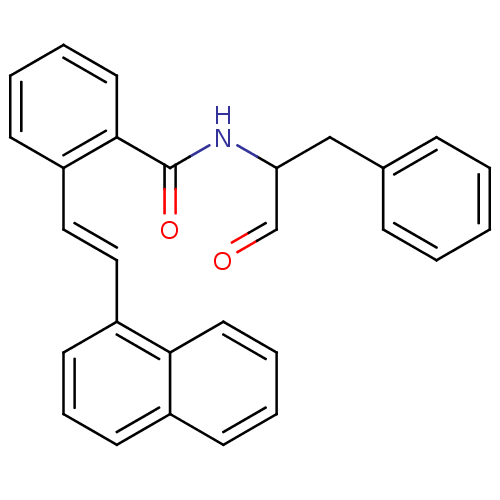
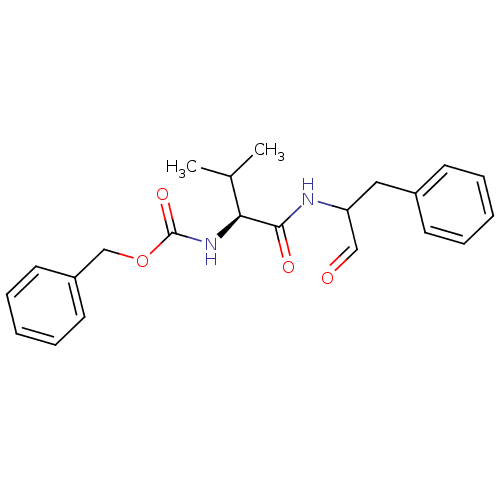
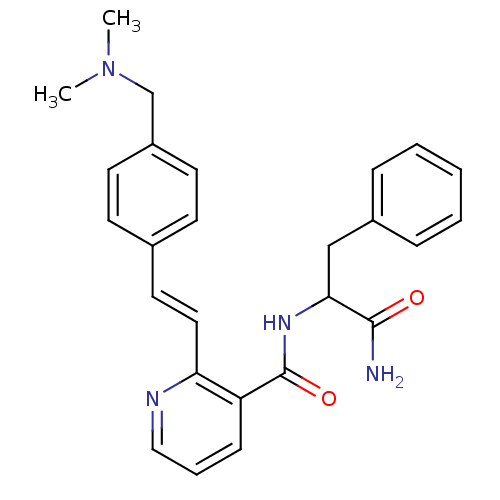
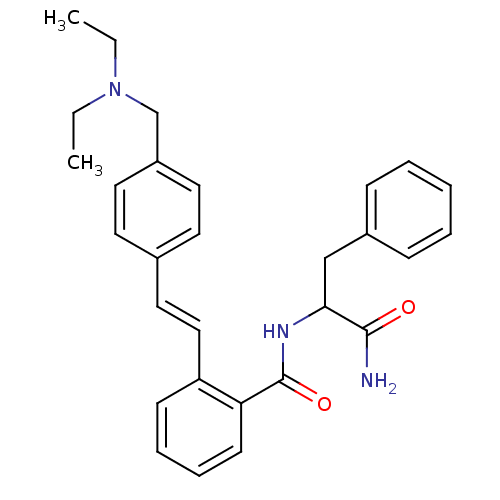

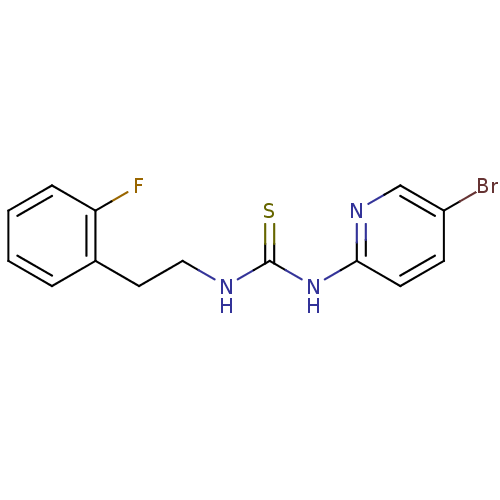
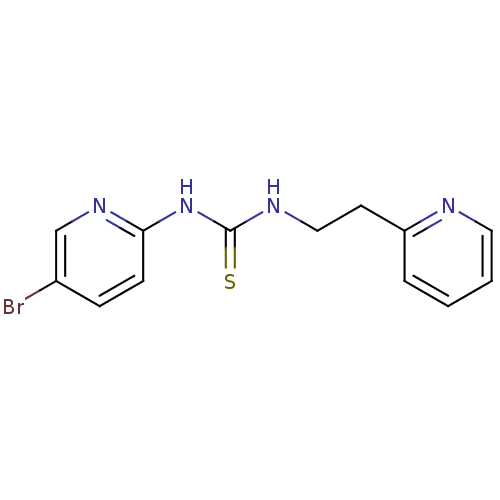
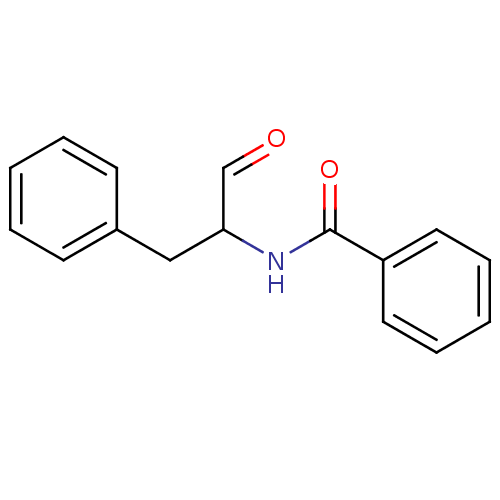

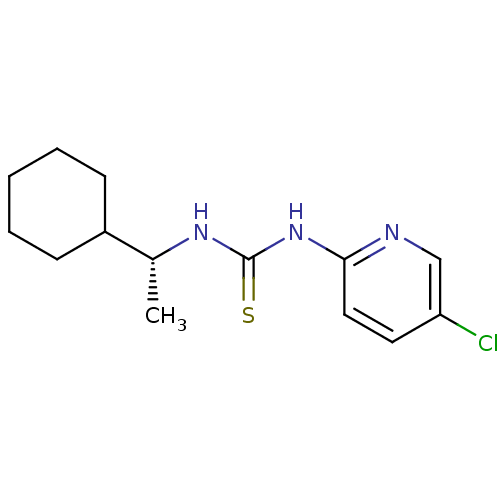
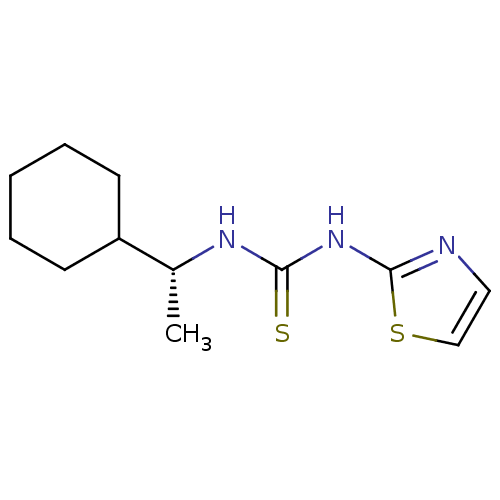
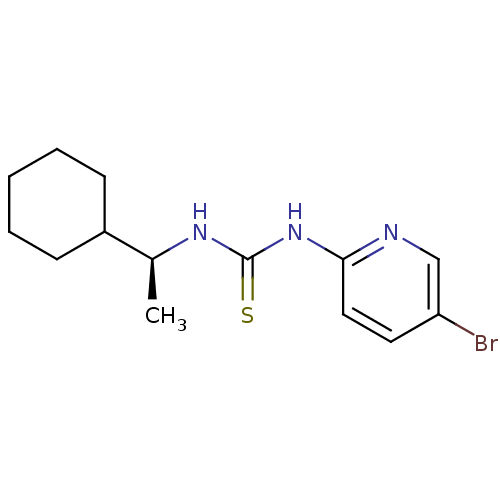
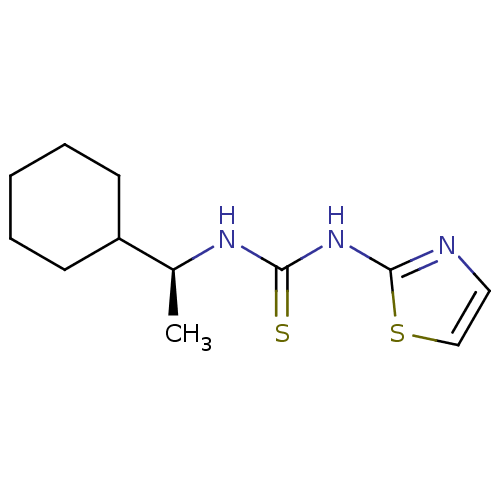
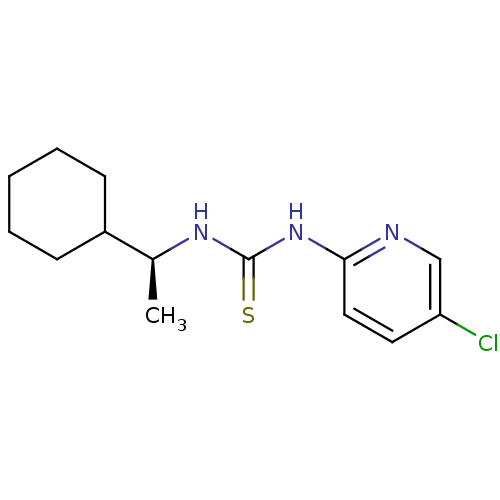
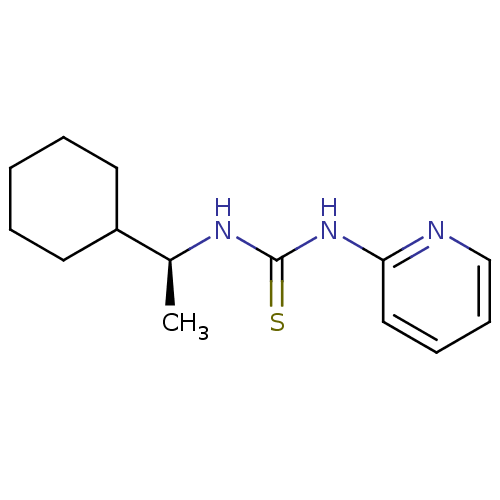
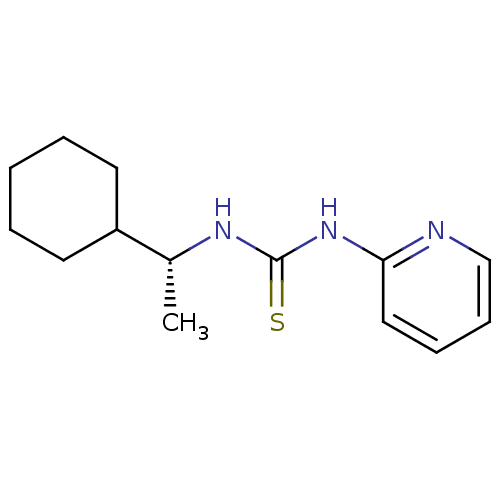
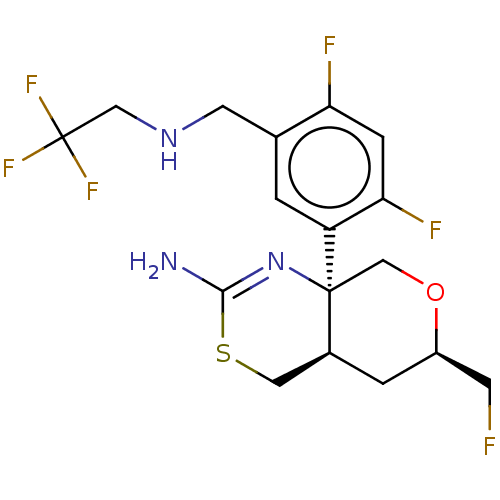
 3D Structure (crystal)
3D Structure (crystal)