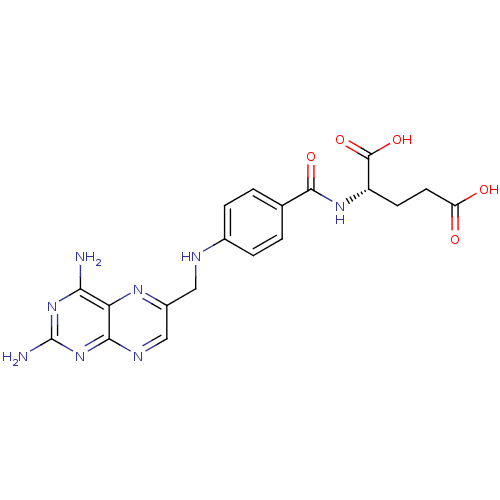
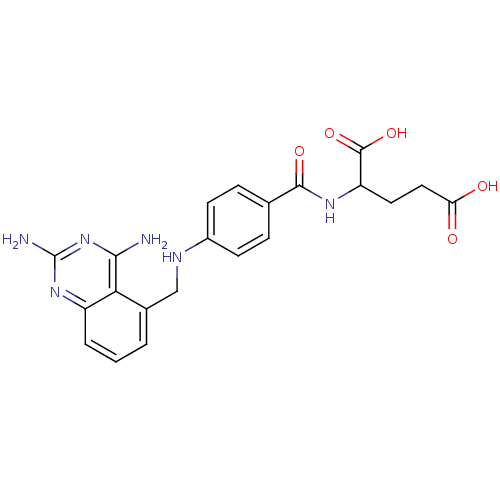
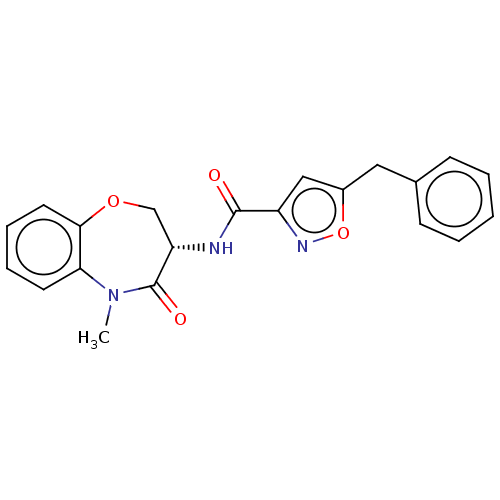
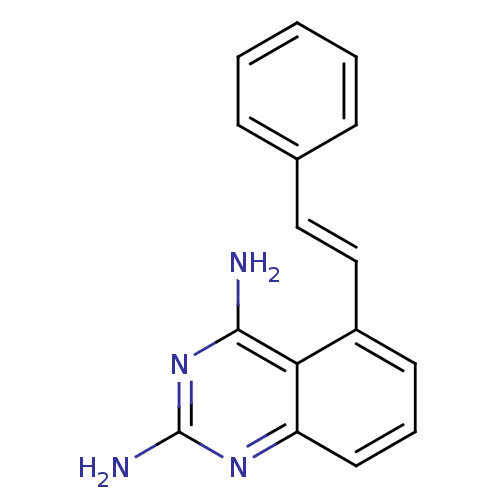
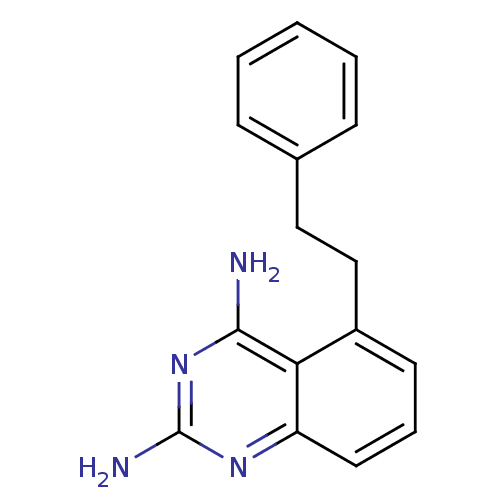
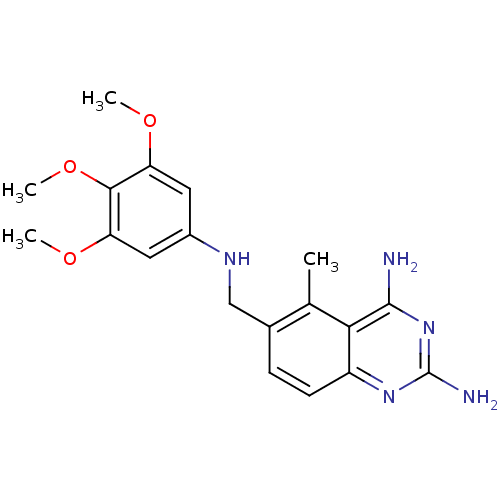
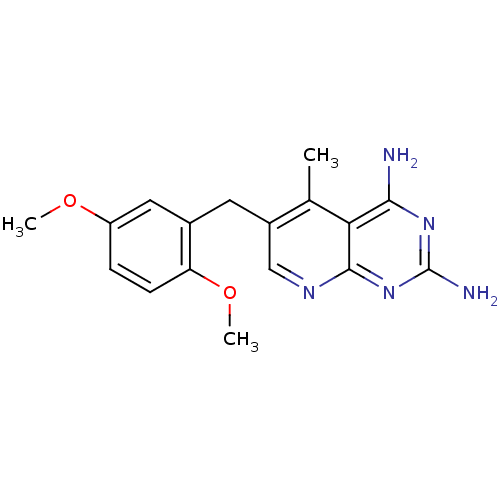
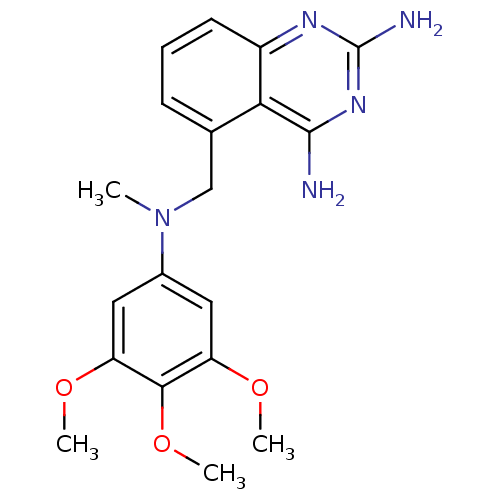
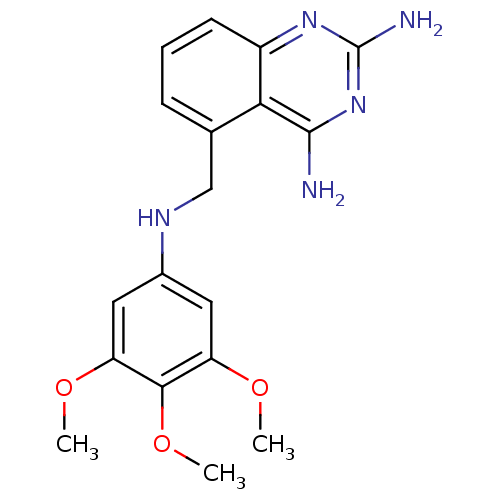
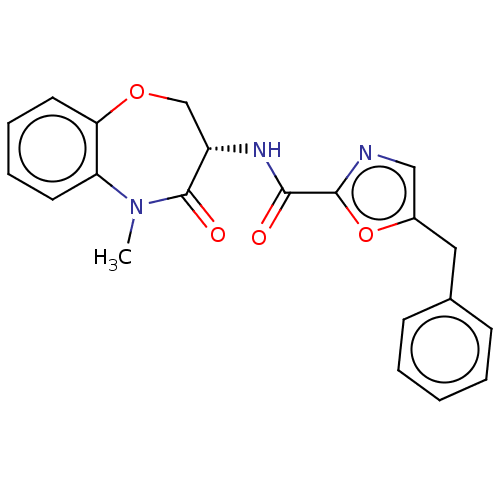
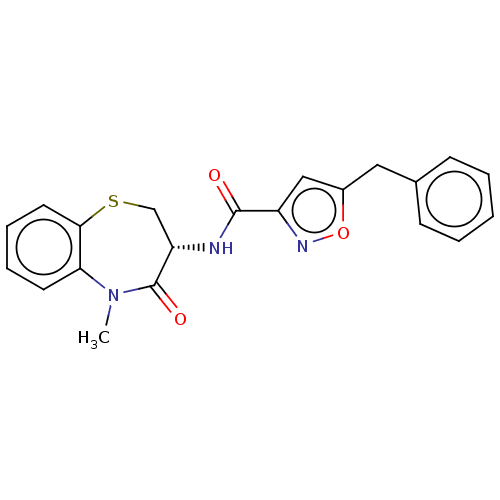
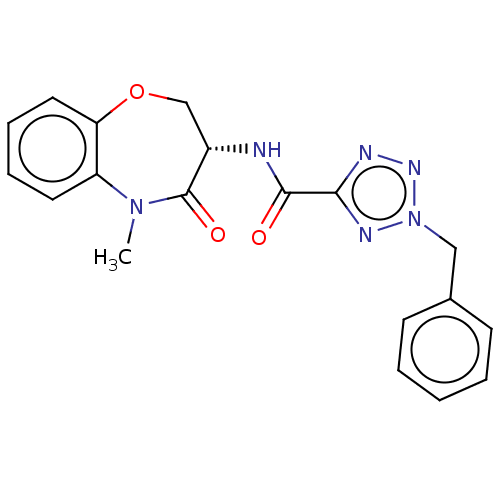
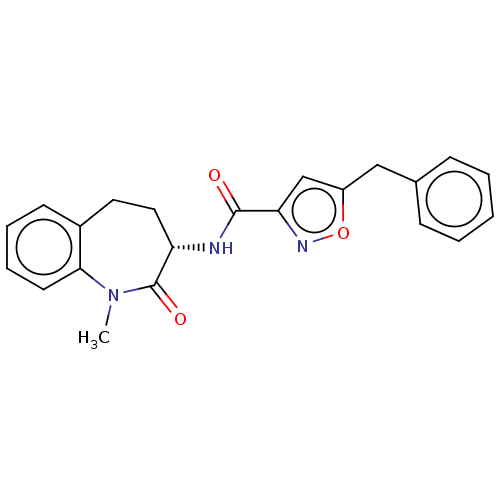
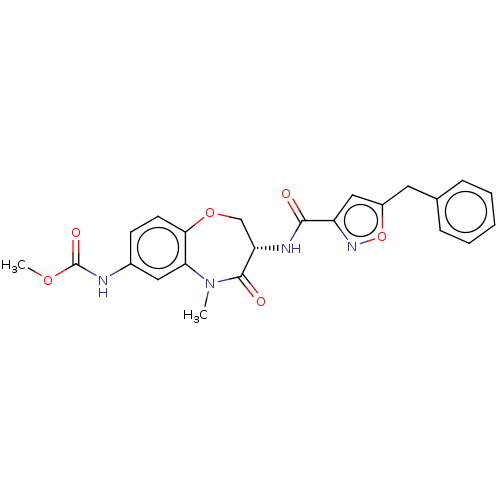
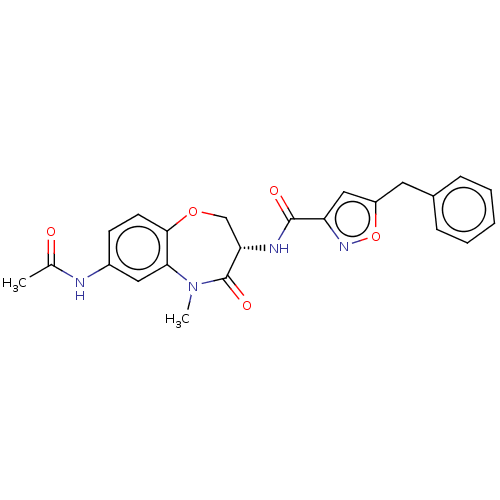
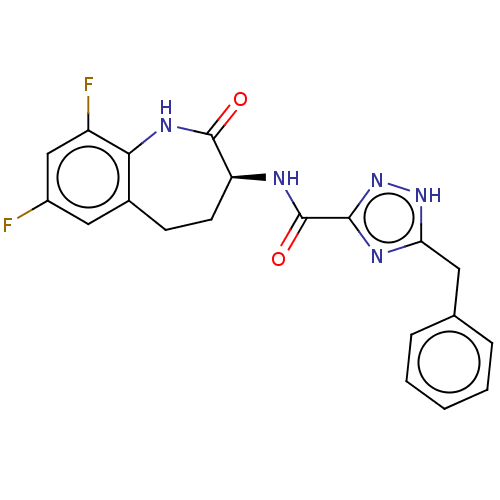
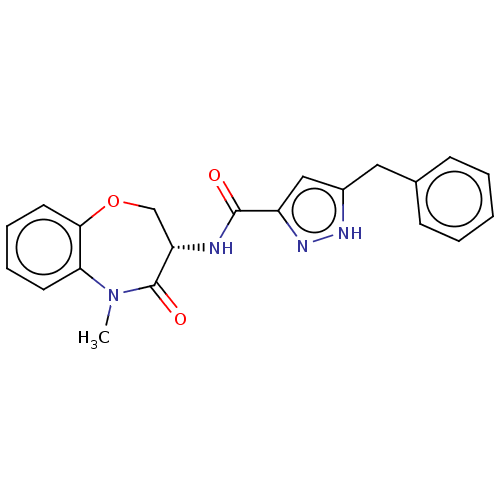
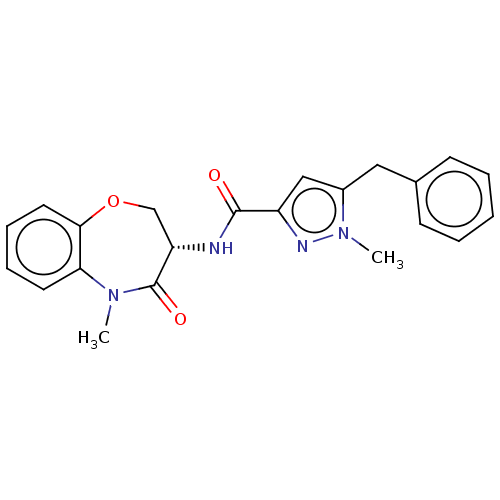
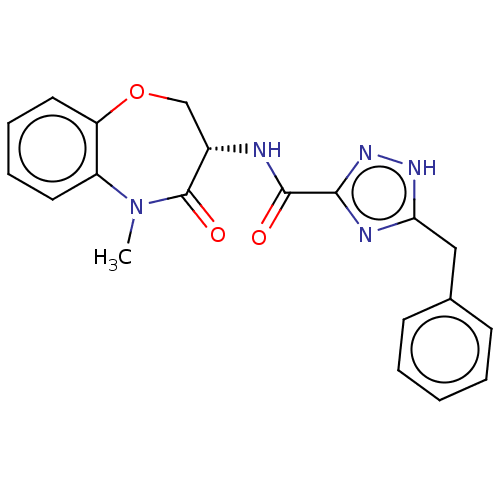
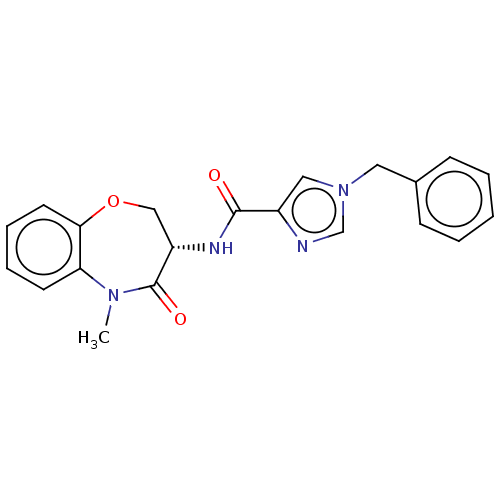
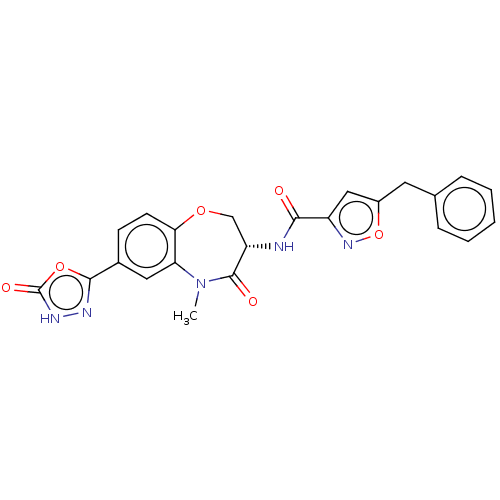
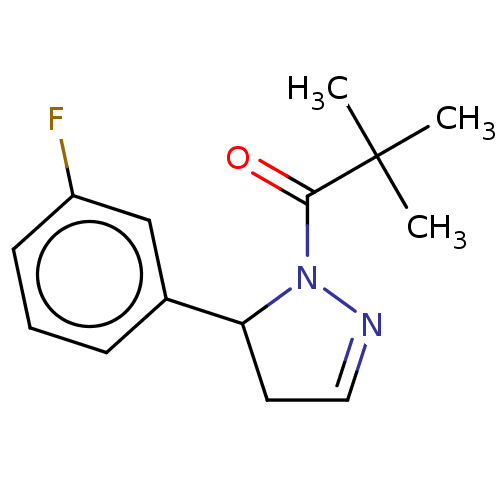
Affinity DataKi: 0.00120nMAssay Description:Inhibitory activity against Wild-type human DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.0100nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.110nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.210nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Inhibitory activity against Wild-type human DHFRMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Competitive inhibition of human RIP1 (1 to 375 residues) in presence of increasing ATP by ADP-Glo reagent based assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.70nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 2.90nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.40nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 13nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 33nMAssay Description:Inhibitory activity against Wild-type human DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 38nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 72nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 83nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 96nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 200nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: 310nMAssay Description:Inhibitory activity against Leu22-Phe mutant human Dihydrofolate reductaseMore data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Inhibitory activity against Wild-type human DHFRMore data for this Ligand-Target Pair
Affinity DataKi: >500nMAssay Description:Inhibitory compound against Wild-type human DHFR.More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.0500nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.0630nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.0790nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.200nMAssay Description:Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assayMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.320nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta mRNA overexpression measured at 21 ...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated MIP-1 beta cytokine overproduction measured at...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of Flag-tagged human RIP1 (1 to 324 residues) after 30 mins by ADP-Glo reagent based assayMore data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-induced necroptosis at 21 hrs post-st...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.400nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of RIP1 in human primary neutrophils assessed as reduction in TNFalpha/QCD-OPh/SMAC mimetic RMT 5265-MIP-1beta production at 21 hrs post-s...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human RIP1 (1 to 375 residues) expressed in baculovirus infected insect cells preincubated for 1 hr followed by ATP addition measured a...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human GST/His-tagged RIP1 (1 to 375 residues) expressed in baculovirus expression system assessed as reduction in autophosphorylation m...More data for this Ligand-Target Pair
TargetReceptor-interacting serine/threonine-protein kinase 1(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 0.5nMAssay Description:Inhibition of RIP1 in human neutrophils assessed as inhibition of TNF-alpha/QVD-Oph/RMT5265-stimulated necroptosis by measuring LDH release measured ...More data for this Ligand-Target Pair

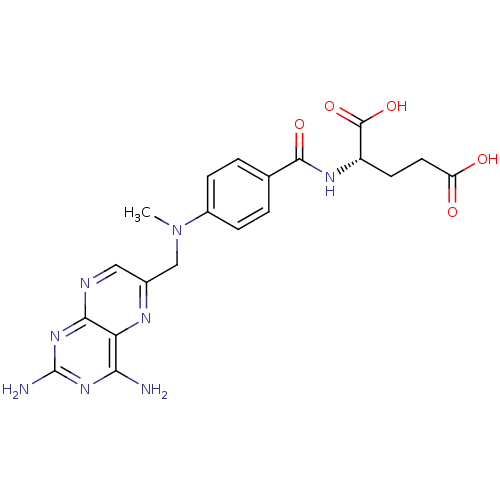
 3D Structure (crystal)
3D Structure (crystal)