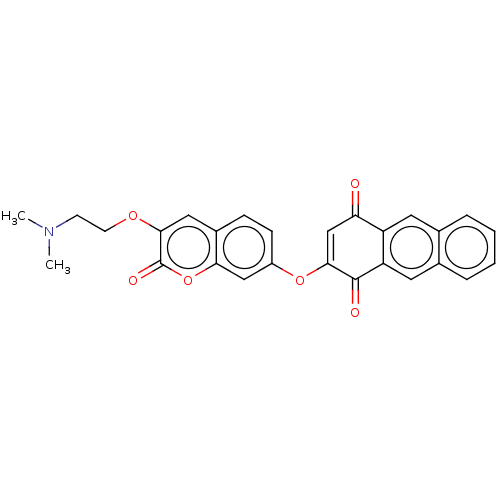
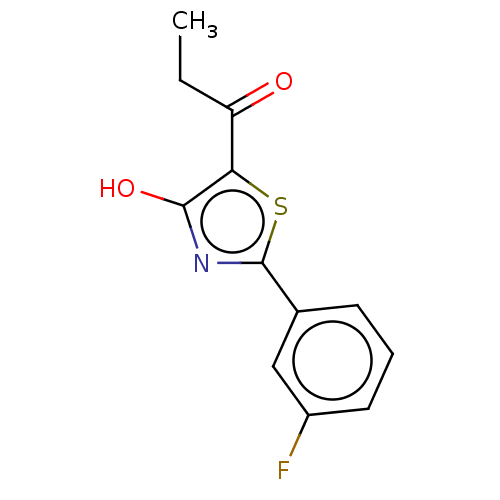
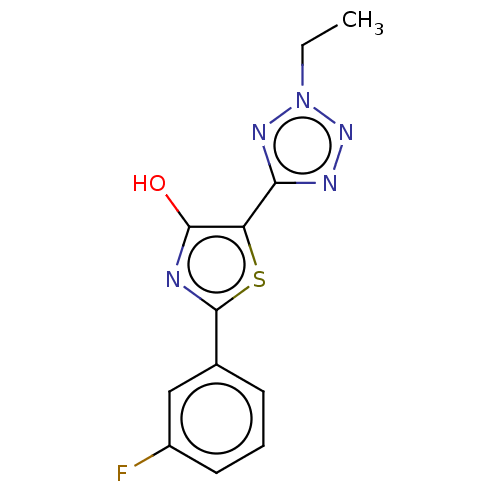
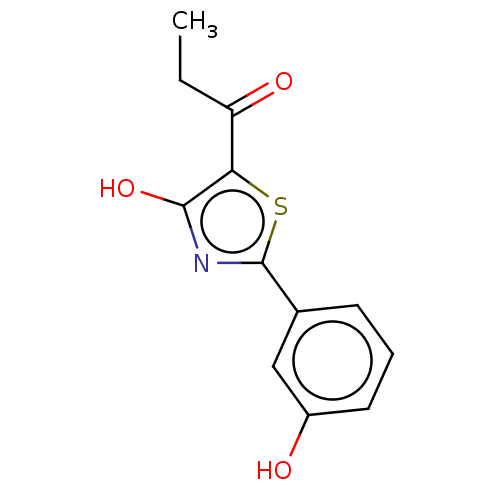
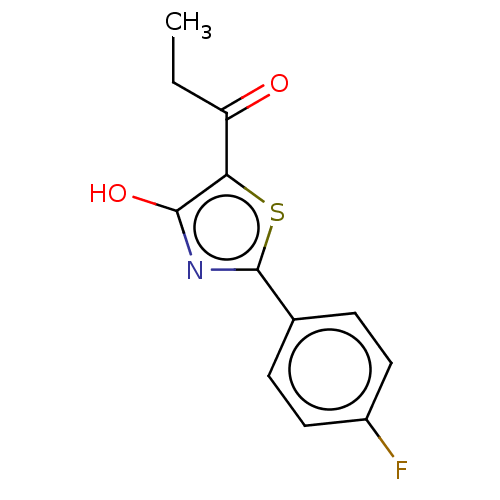
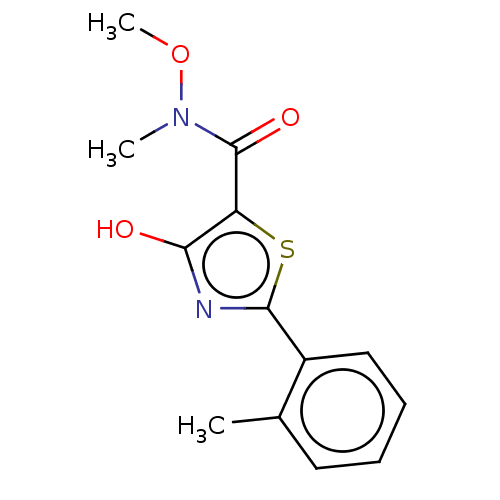
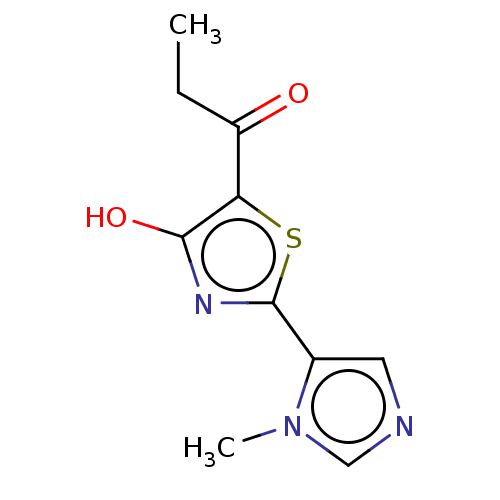
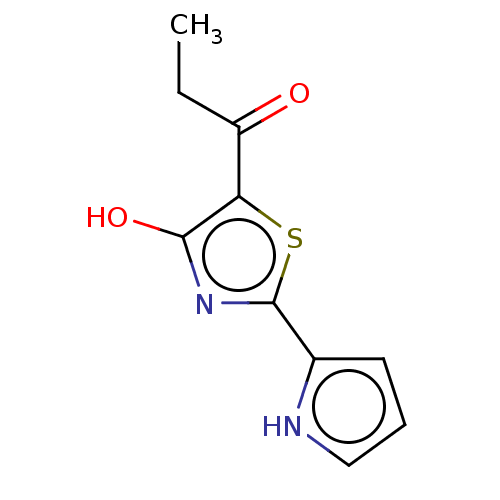
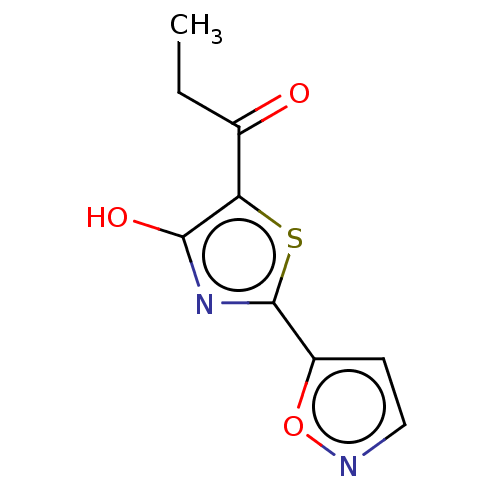
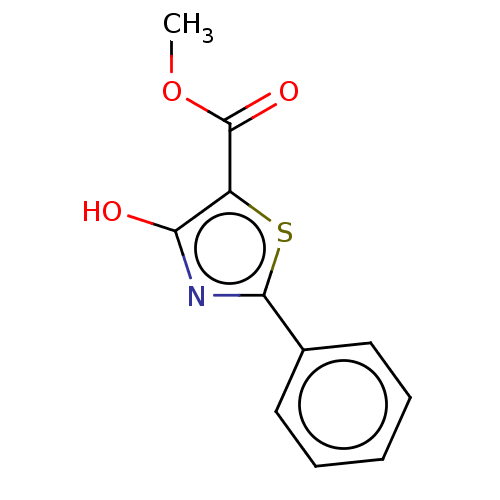
Affinity DataKi: 2.30E+3nMAssay Description:Displacement of 125-I echistatin from Vitronectin receptor (alpha v beta3)More data for this Ligand-Target Pair
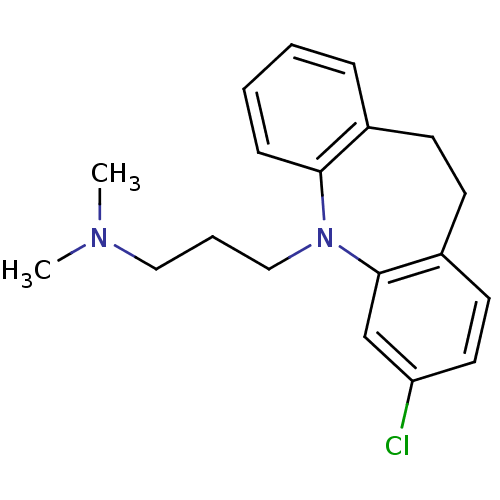
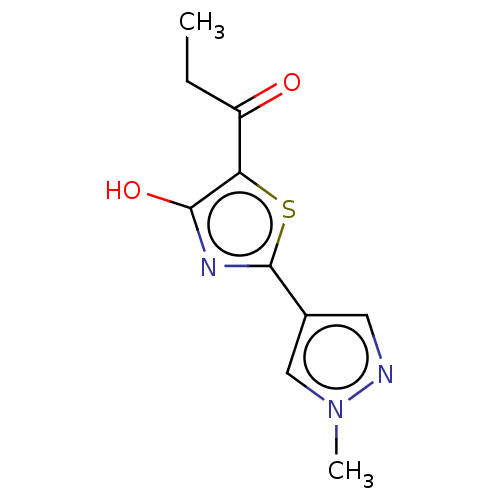
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as...More data for this Ligand-Target Pair
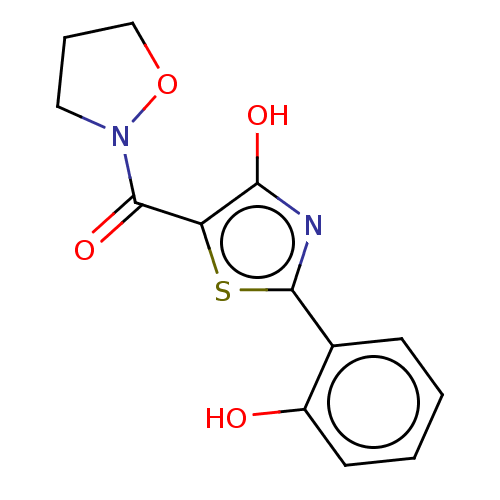
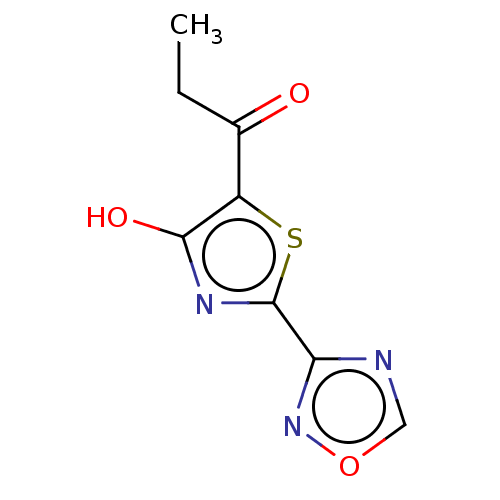
Affinity DataKi: 5.60E+3nMAssay Description:Mixed-type inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione d...More data for this Ligand-Target Pair
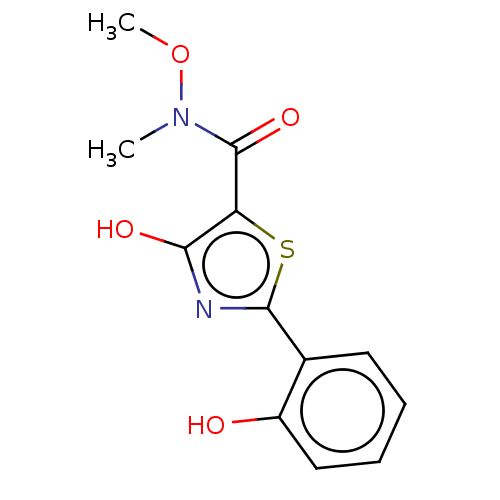
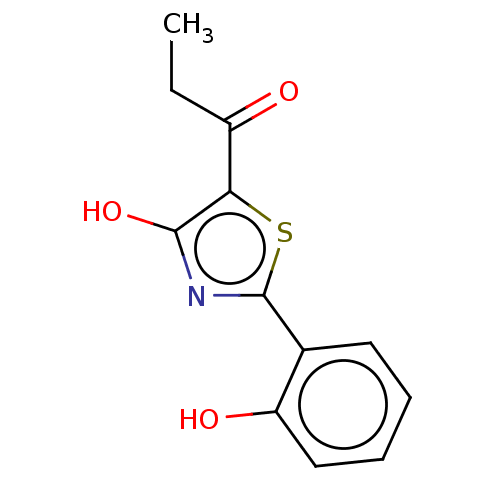
Affinity DataKi: 6.50E+3nMAssay Description:Inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothione disulfide as...More data for this Ligand-Target Pair
Affinity DataKi: 2.24E+4nMAssay Description:Non-competitive inhibition of Trypanosoma cruzi trypanothione reductase assessed as reduction in NADPH consumption using varying levels of trypanothi...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ efflux preincubated for 5 mins followed by icilin ad...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 5.5nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in cold stimulated Ca2+ efflux preincubated for 5 mins followed by cold sti...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 8.5nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in cold stimulated Ca2+ efflux preincubated for 5 mins followed by cold sti...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 12nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in cold stimulated Ca2+ efflux preincubated for 5 mins followed by cold sti...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 14nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in cold stimulated Ca2+ efflux preincubated for 5 mins followed by cold sti...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 16nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 17nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ efflux preincubated for 5 mins followed by icilin ad...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 18nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 21nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in cold stimulated Ca2+ efflux preincubated for 5 mins followed by cold sti...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 24nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 24nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ efflux preincubated for 5 mins followed by icilin ad...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 29nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 32nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Affinity DataIC50: 33nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ efflux preincubated for 5 mins followed by icilin ad...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as decrease in outward currents by patch clamp electrophysiology methodMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 39nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 50nMAssay Description:Inhibition of human TRPM8 I746A mutant expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 60nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 60nMAssay Description:Inhibition of human TRPM8 I746A mutant expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ effluxMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 61nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 66nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily M member 8(Rattus norvegicus (Rat))
National Research Council
Curated by ChEMBL
National Research Council
Curated by ChEMBL
Affinity DataIC50: 72nMAssay Description:Antagonist activity at recombinant rat TRPM8 expressed in HEK293 cells assessed as inhibition of icilin-induced intracellular calcium accumulation pr...More data for this Ligand-Target Pair
Affinity DataIC50: 75nMAssay Description:Inhibition of wild type human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ ef...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 76nMAssay Description:Inhibition of wild type human TRPM8 expressed in HEK293 cells assessed as reduction in icilin induced Ca2+ effluxMore data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 80nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 81nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 96nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Affinity DataIC50: 131nMAssay Description:Inhibition of human TRPM8 expressed in HEK293 cells assessed as reduction in ethyl 4-methoxy-2-phenylthiazole-5-carboxylate induced Ca2+ efflux prein...More data for this Ligand-Target Pair
Ligand Info