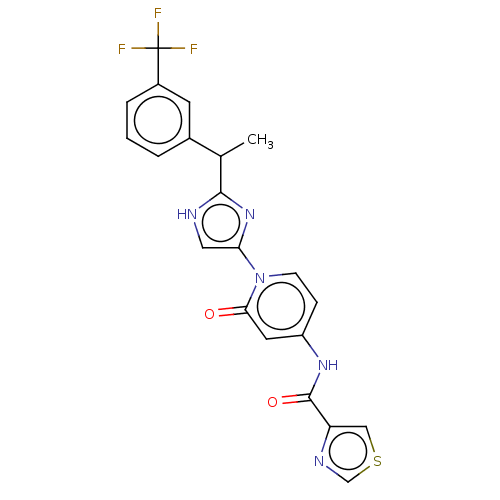
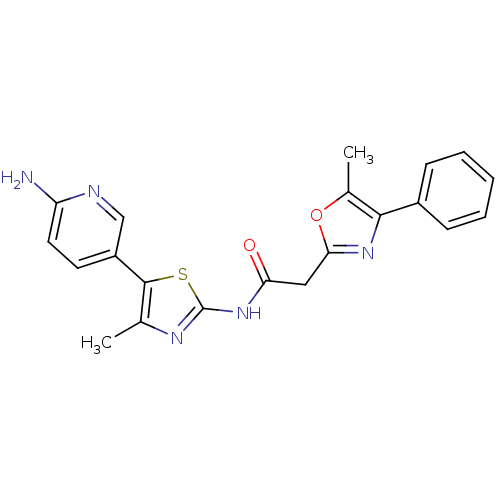
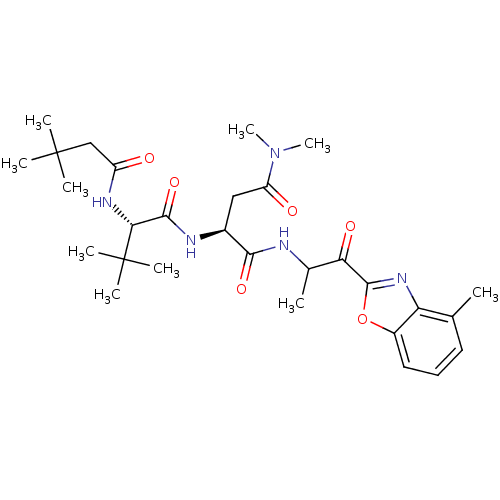
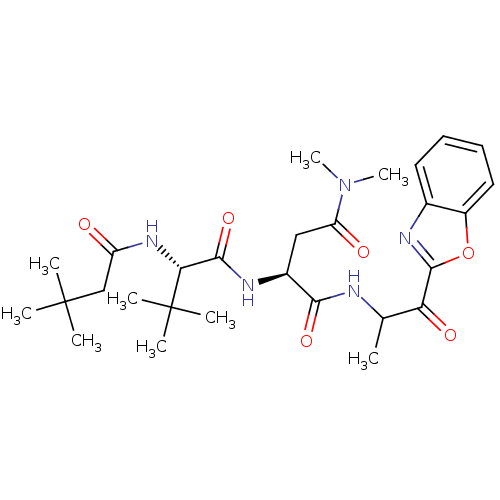
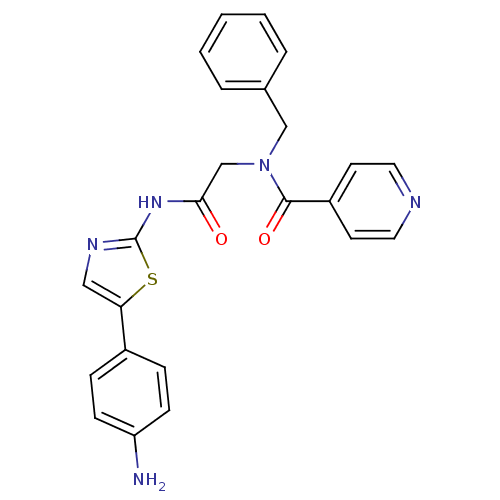
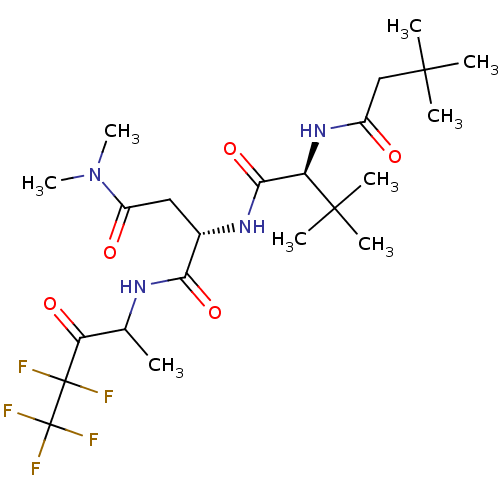
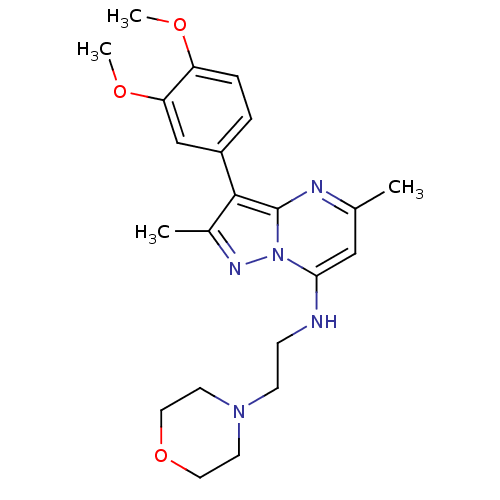
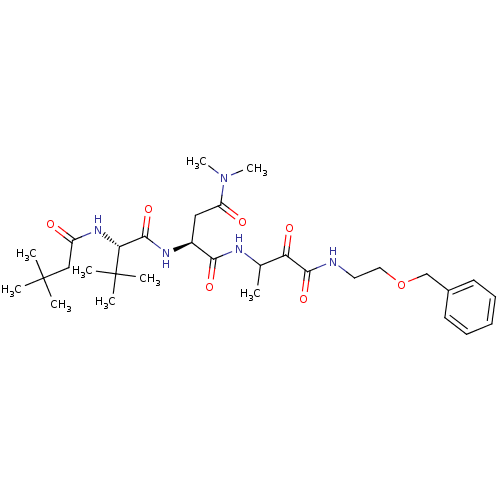
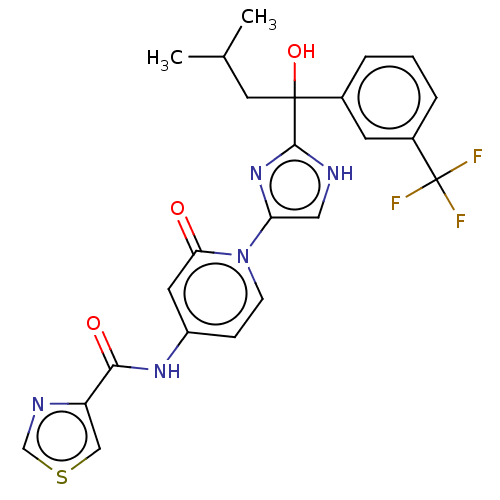
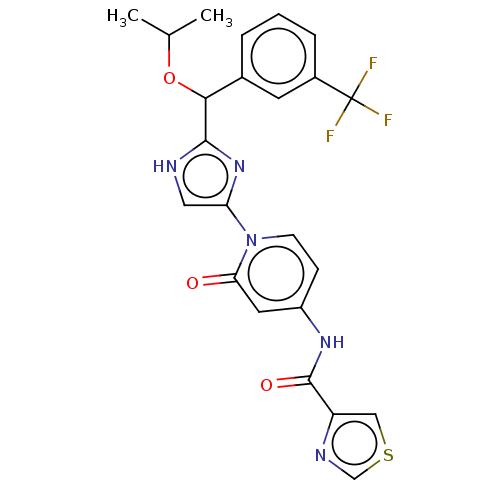
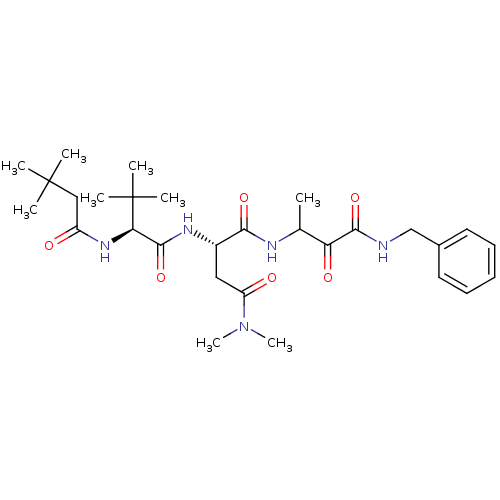
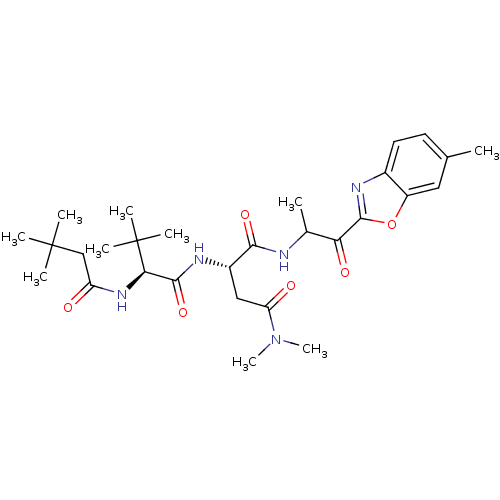
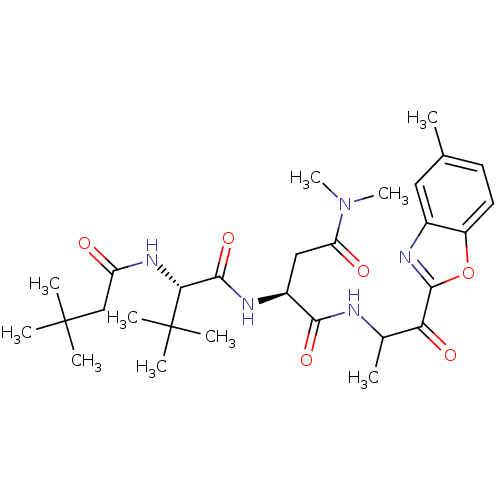
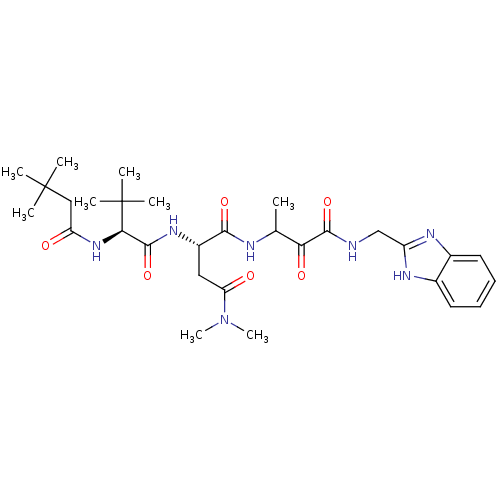
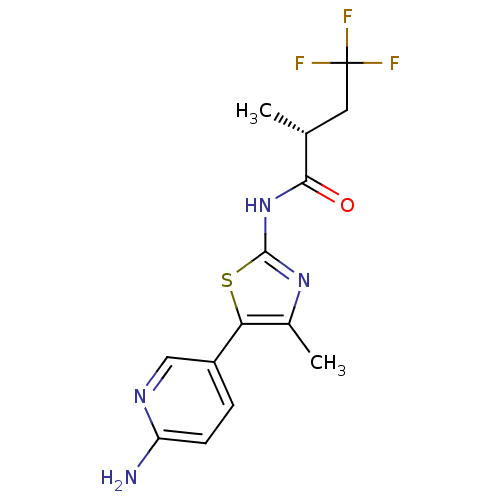
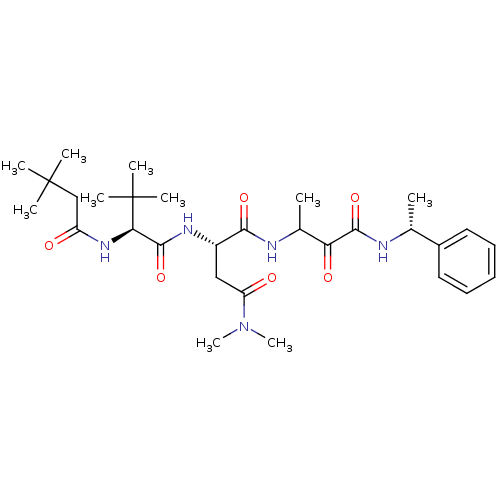
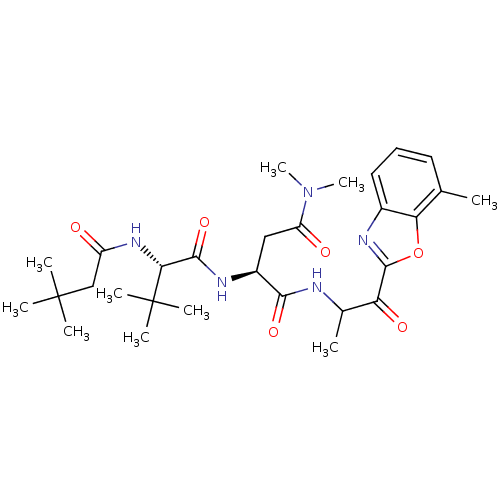
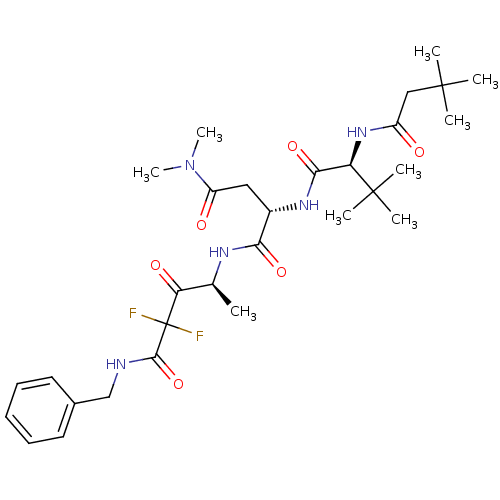
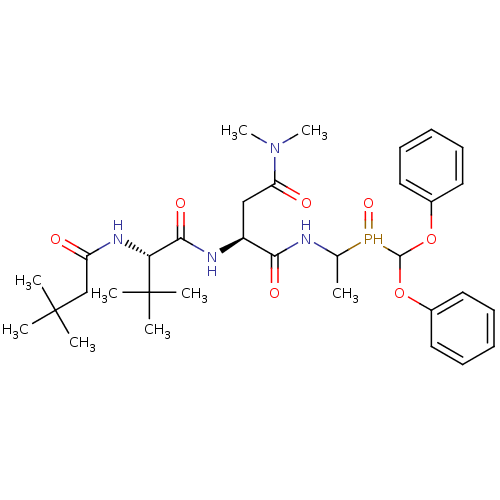
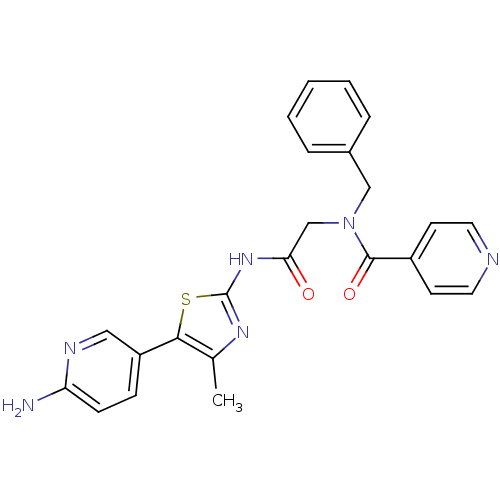
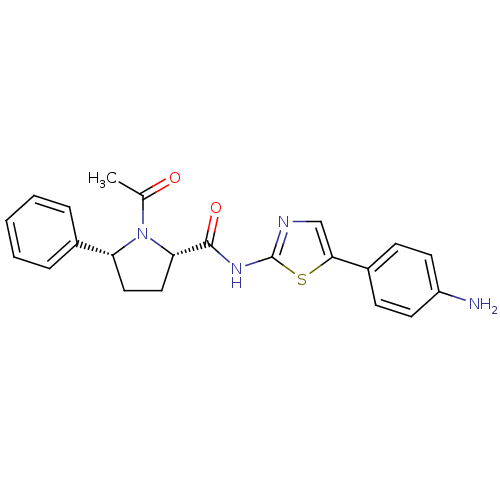
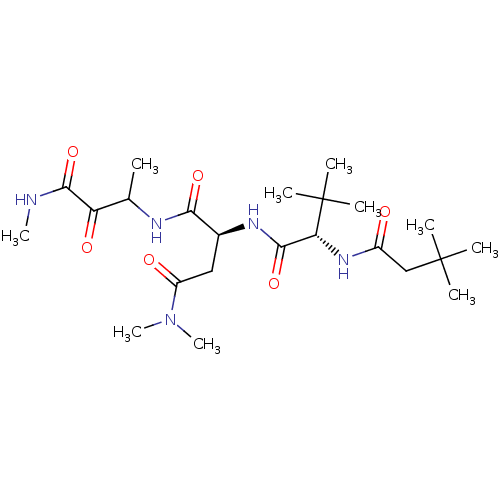
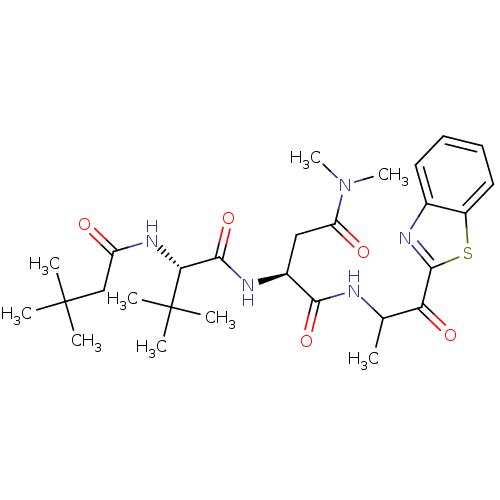
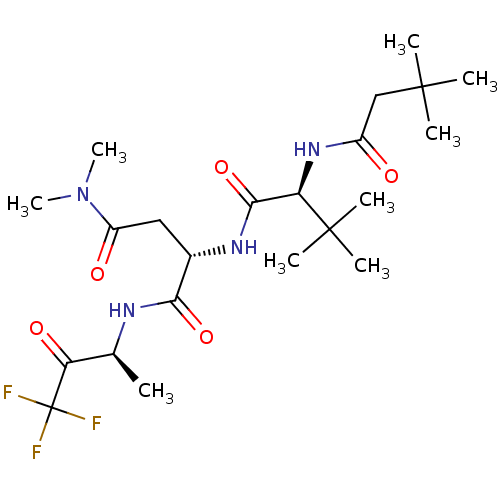
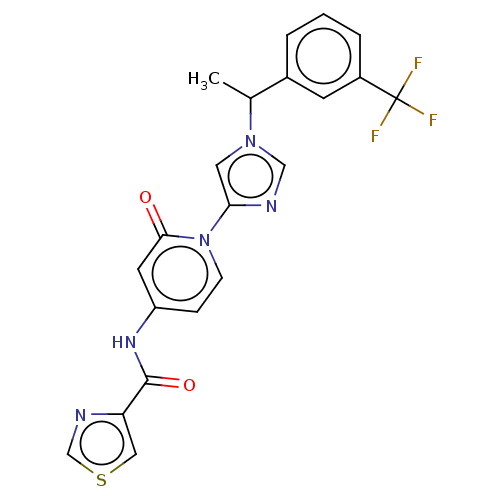
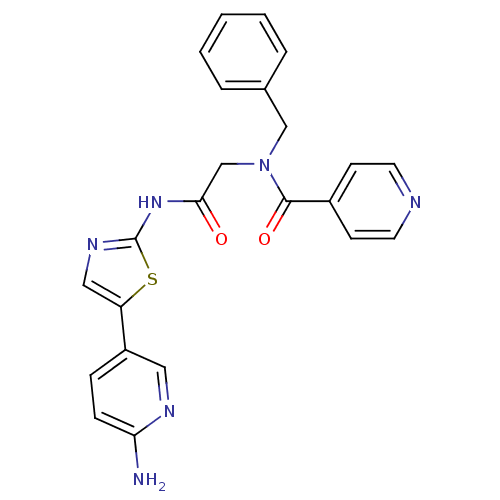
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 22nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
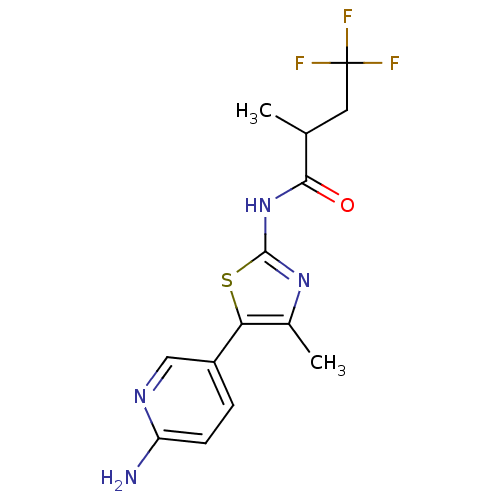
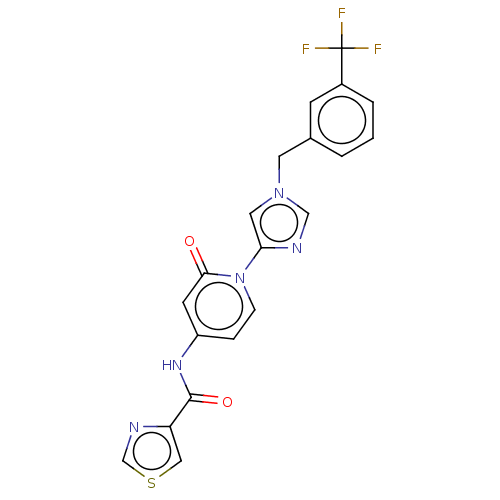
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 35nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
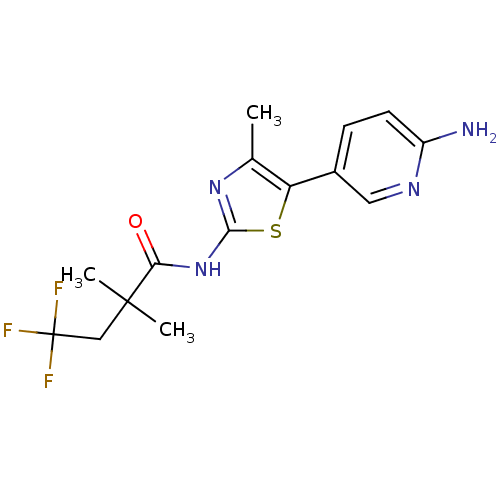
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 40nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
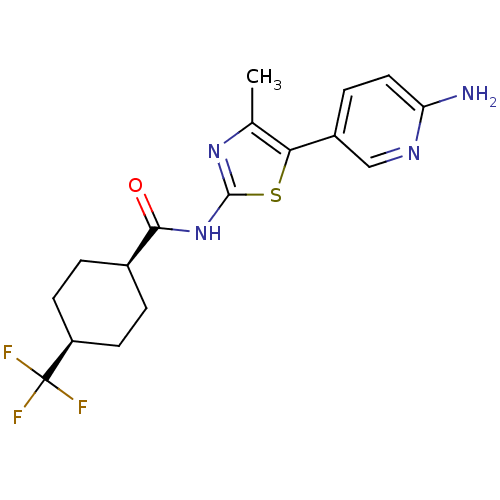
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 48nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 53nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 67nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:Activity against serine protease porcine pancreatic elastase (PPE)More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Activity against serine protease porcine pancreatic elastase (PPE)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 137nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 180nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Activity against serine protease porcine pancreatic elastase (PPE)More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Activity against serine protease porcine pancreatic elastase (PPE)More data for this Ligand-Target Pair
Affinity DataIC50: 210nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4-kinase beta(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 225nMAssay Description:Inhibition of PI4K3beta (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Activity against serine protease porcine pancreatic elastase (PPE)More data for this Ligand-Target Pair
Affinity DataIC50: 460nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 500nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 660nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 710nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 980nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.10E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+3nMAssay Description:Inhibition of CYP2C8 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Micro molar potency of the compound to inhibit human cytomegalovirus (HCMV) proteaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase 17A(Homo sapiens (Human))
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Boehringer Ingelheim (Canada)
Curated by ChEMBL
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of STK17A (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+3nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair