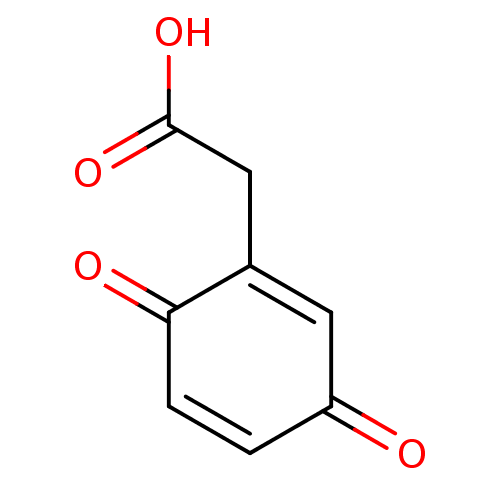
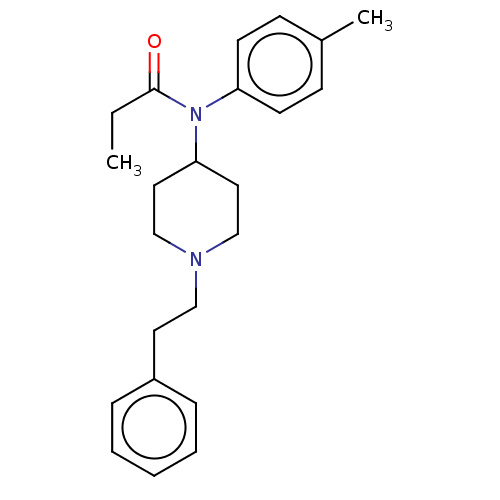
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 460nMAssay Description:Competitive inhibition of Clostridium botulinum neurotoxin serotype A light chainMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 895nMAssay Description:Uncompetitive inhibition of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 fol...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 2.03E+3nMAssay Description:Uncompetitive inhibition of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for 20 fol...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 7.70E+3nMAssay Description:Inhibition of Clostridium botulinum neurotoxin serotype A light chainMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as reduction in benzoquinone-mediated BoNT/A i...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.55E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as reduction in initial rates of BoNT/A LC act...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.30E+3nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.50E+3nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.26E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.36E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.66E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.28E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.39E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.19E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 7.32E+3nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.30E+3nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.27E+4nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.28E+4nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.40E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.80E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.83E+4nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.50E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.86E+4nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.76E+4nMAssay Description:Inhibition of protease activity of recombinant Clostridium botulinum BoNT/A light chain (425 amino acids) using SNAP-25 as substrate preincubated for...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.80E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 9.70E+4nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.20E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.60E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 1.63E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as reduction in initial rates of BoNT/A LC act...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.00E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.10E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 2.58E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as reduction in benzoquinone-mediated BoNT/A i...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.17E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.70E+5nMAssay Description:Competitive inhibition of recombinant Clostridium botulinum neurotoxin serotype A light chain assessed as [13C]-labeled 9-mer cleavage product format...More data for this Ligand-Target Pair
Affinity DataKd: 0.143nM Kon: 0.0210M-1s-1 Koff: 1.47E+7s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 0.737nM Kon: 0.00479M-1s-1 Koff: 6.50E+6s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 0.194nM Kon: 0.00213M-1s-1 Koff: 1.10E+7s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 6.92nM Kon: 0.0972M-1s-1 Koff: 1.41E+7s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 0.219nM Kon: 0.00207M-1s-1 Koff: 9.46E+6s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 0.173nM Kon: 0.00173M-1s-1 Koff: 1.00E+7s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair
Affinity DataKd: 0.0718nM Kon: 0.000243M-1s-1 Koff: 3.39E+6s-1Assay Description:The direct binding kinetics of JBZ-4 to fentanyl and related analogues was thoroughly characterized by surface plasmon resonance (SPR) single cycle k...More data for this Ligand-Target Pair