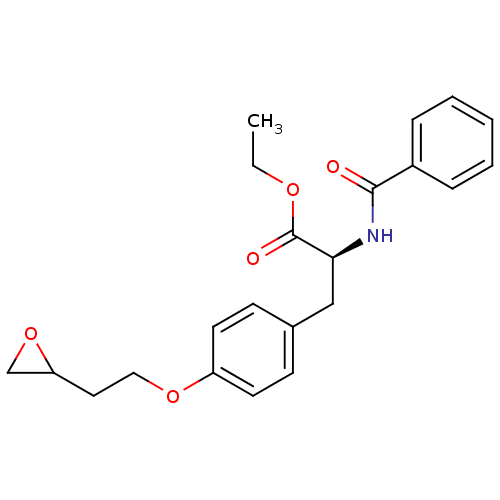
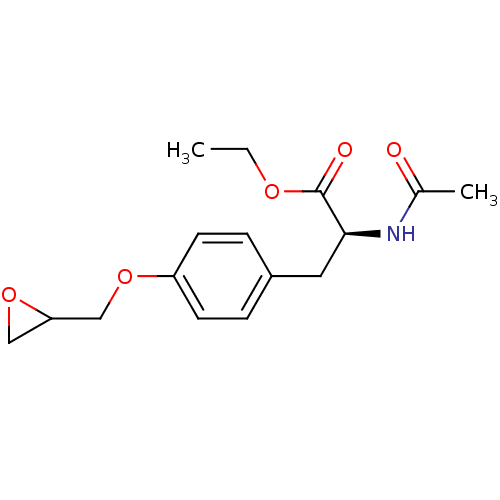
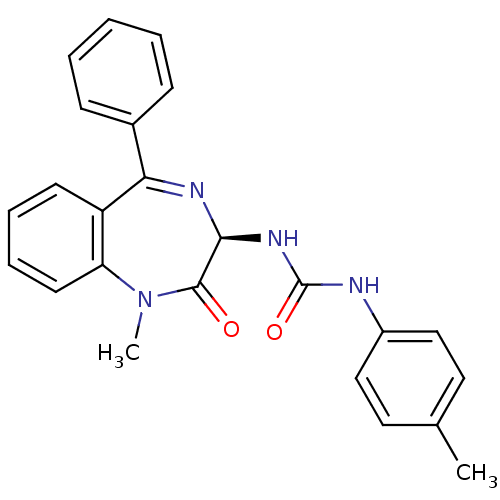
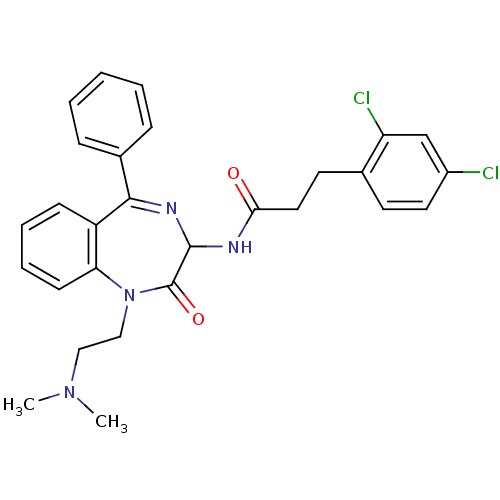
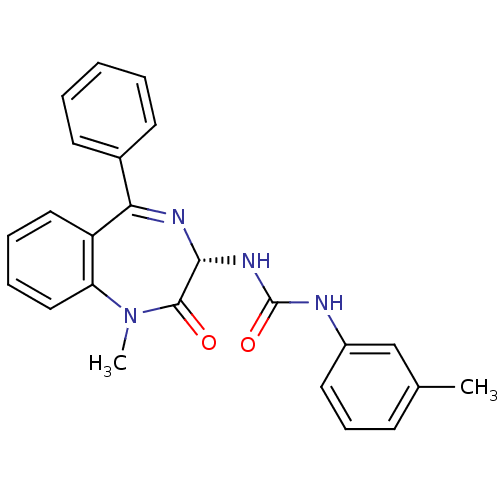
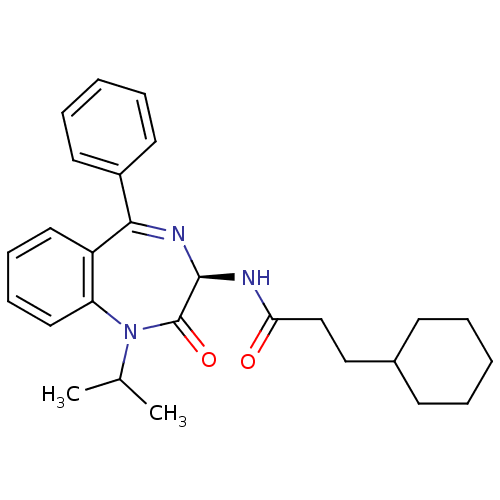
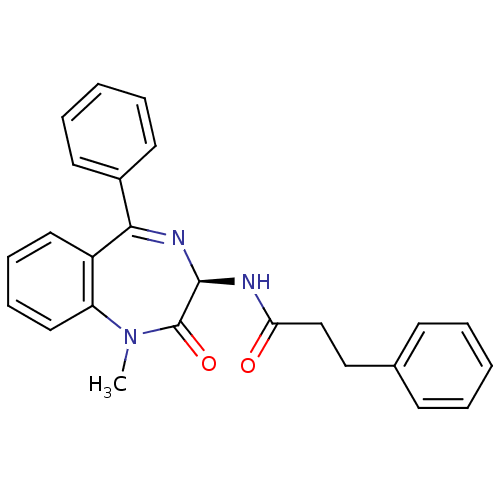
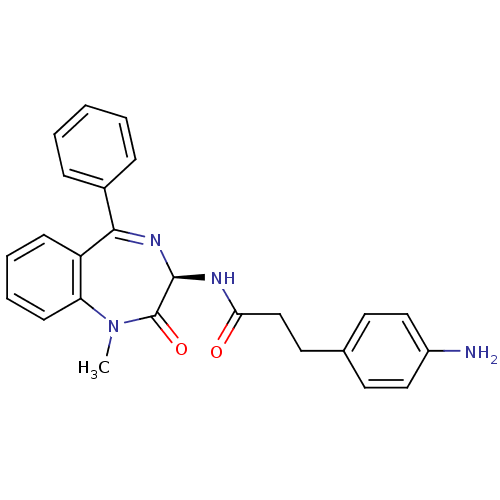
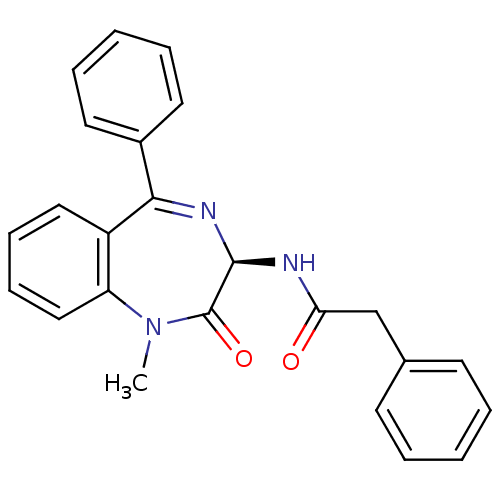
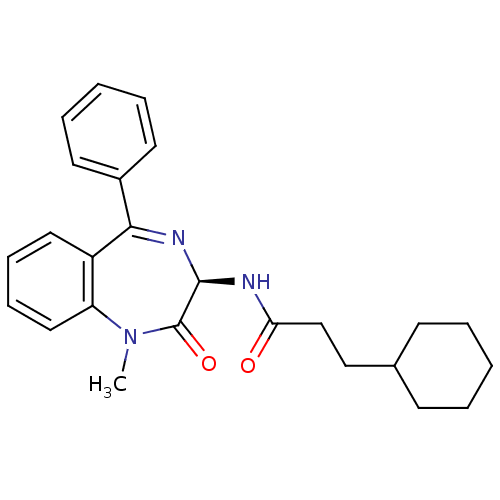
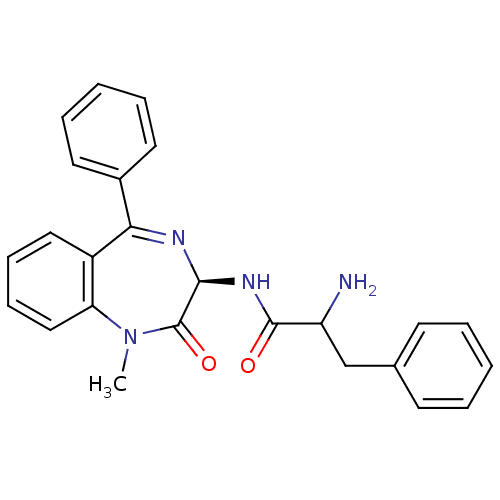
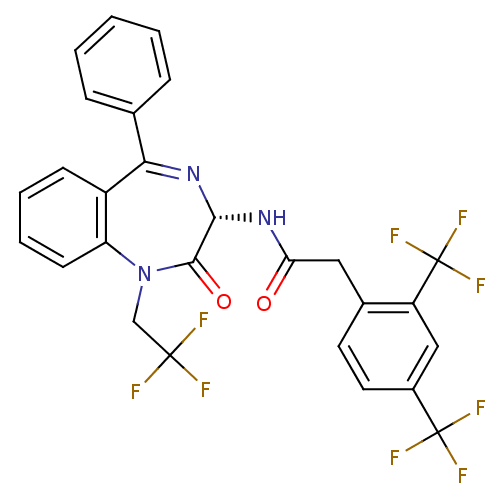
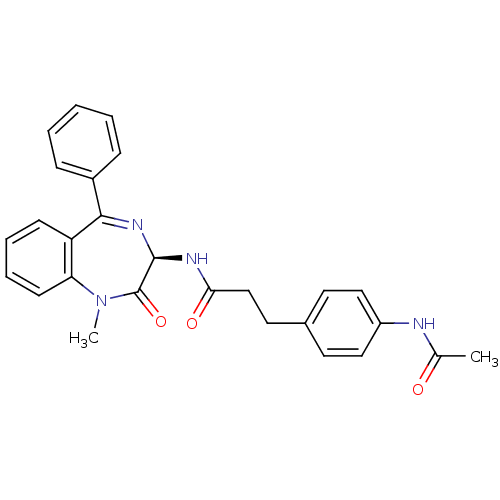
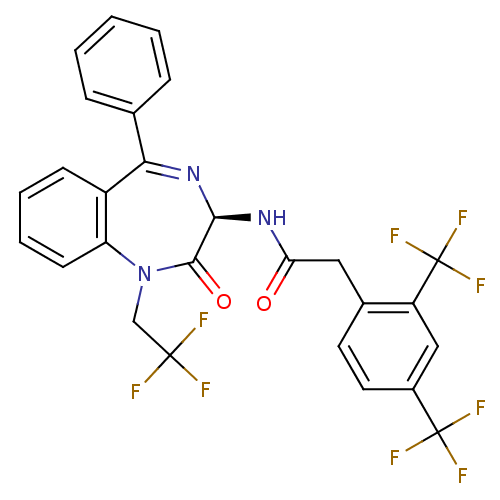
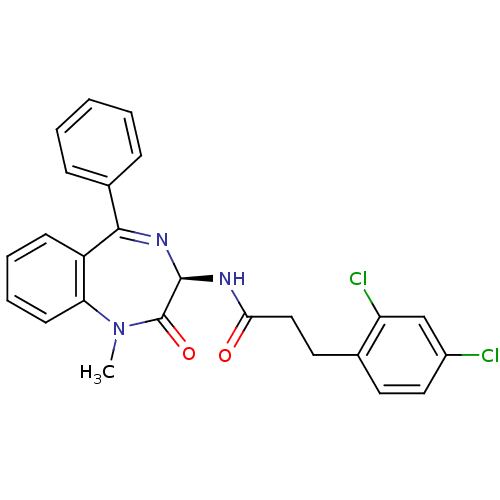
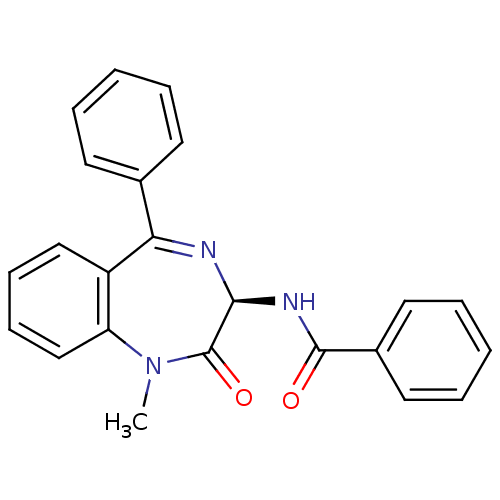
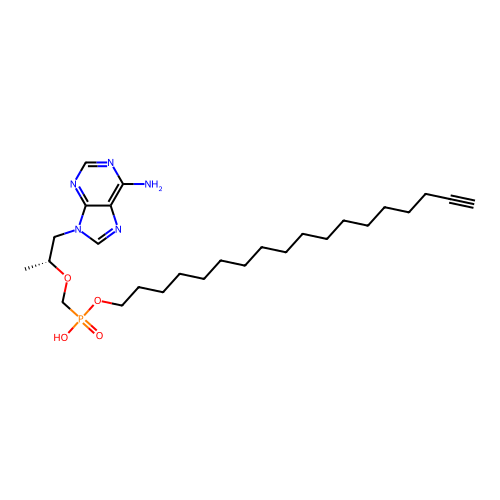
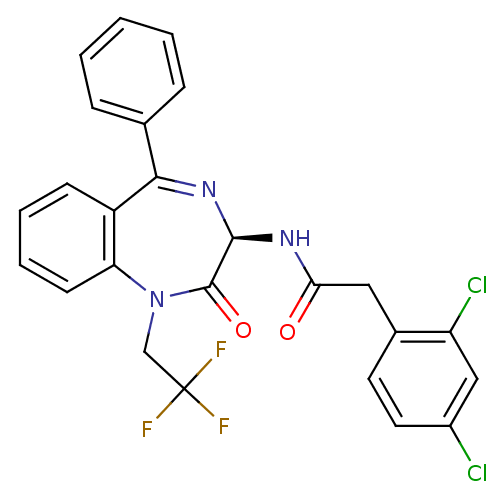
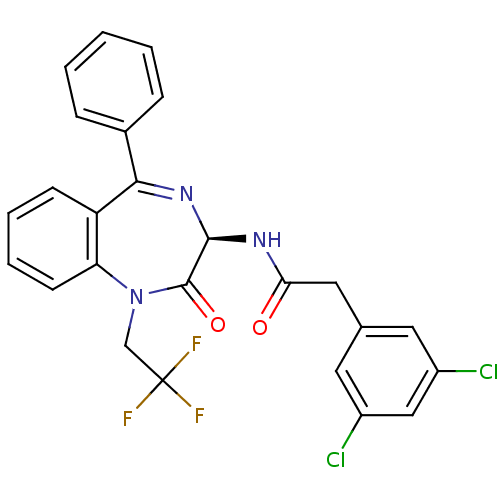
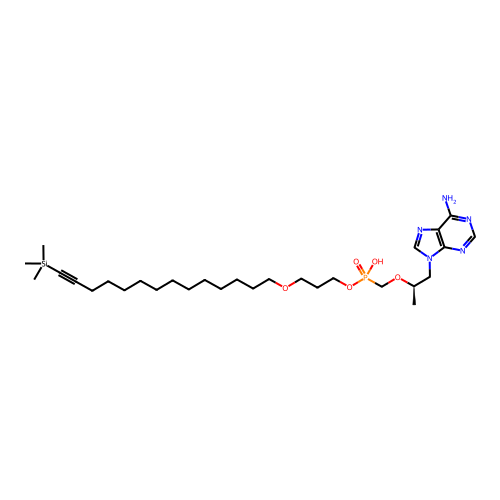
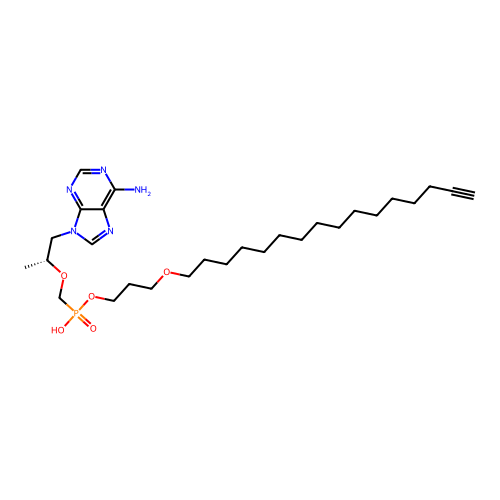
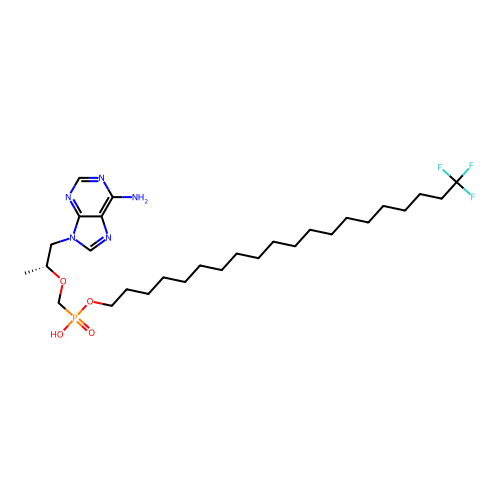
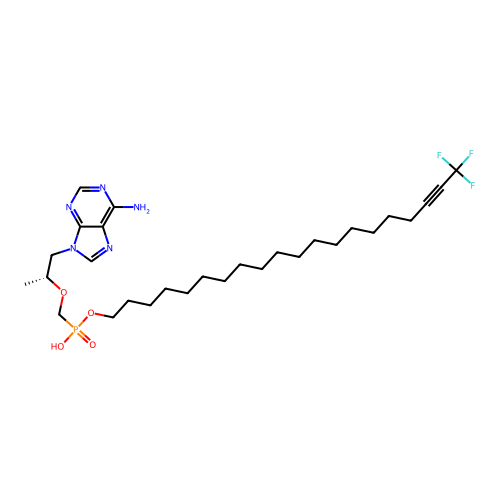
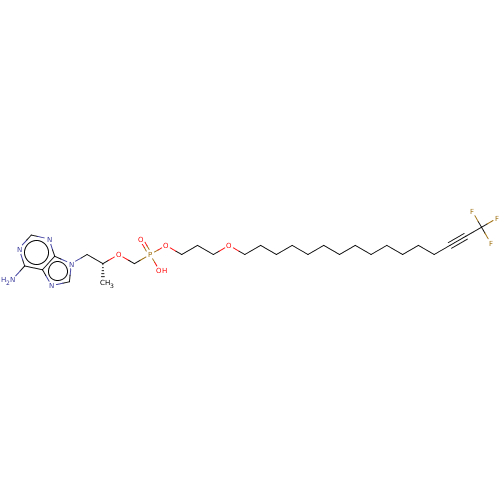Affinity DataKi: 1.40E+3nMAssay Description:Inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.30E+3nMAssay Description:Inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+4nMAssay Description:Inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.38E+4nMAssay Description:Inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.50E+4nMAssay Description:Inhibitory activity of the compound was tested against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.70E+4nMAssay Description:Inhibitory activity of the compound was tested against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 5.90E+4nMAssay Description:Inhibitory activity of the compound was tested against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 6.60E+4nMAssay Description:Inhibitory activity of the compound was tested against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 2.00E+5nMAssay Description:Inhibitory activity of the compound was tested against subtilisinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+5nMAssay Description:Inhibitory activity against alpha-chymotrypsinMore data for this Ligand-Target Pair
Affinity DataKi: 3.80E+5nMAssay Description:Inhibitory activity of the compound was tested against alpha-chymotrypsinMore data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 100nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 151nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetGastrin/cholecystokinin type B receptor(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: >1.00E+3nMAssay Description:Half maximal inhibition of binding of [125I]-CCK-8 to Cholecystokinin type B receptor in guinea pig cerebral cortex.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
Affinity DataIC50: 2.39E+3nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 8.80E+3nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Ikr current in isolated guinea pig myocytes during a 0.5 s voltage clamp step from -50 to -10 mV.More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: 1.87E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP3A4 expressed in insect microsome using 7-benzyloxy-4-trifluoromethylcoumarin as a substrate incubated for 30 mins...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human recombinant CYP2D6 expressed in insect microsome using (3-[2-(N,N-dimethyl-N-methylammonium)-ethyl]-7-methoxy-4-methylcoumarin io...More data for this Ligand-Target Pair