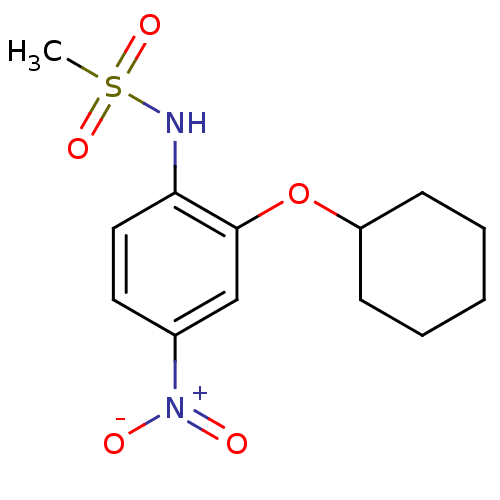
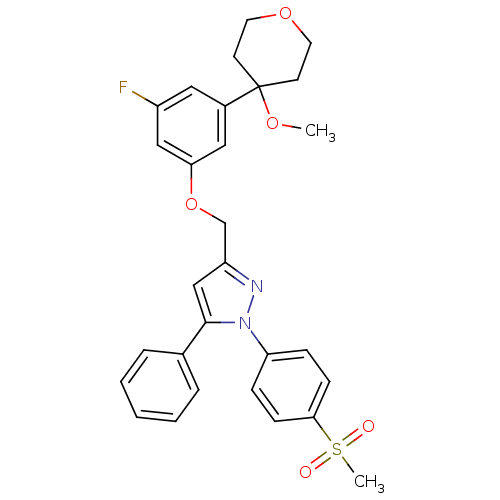
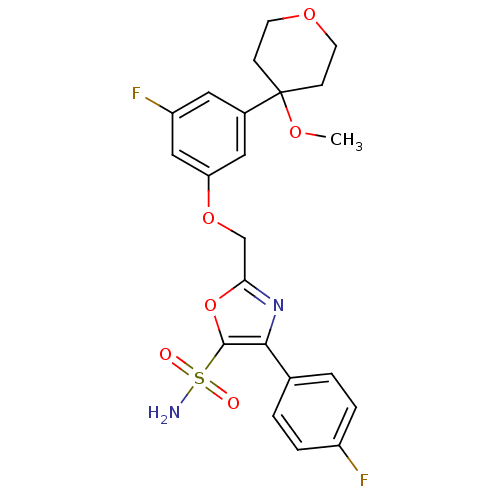
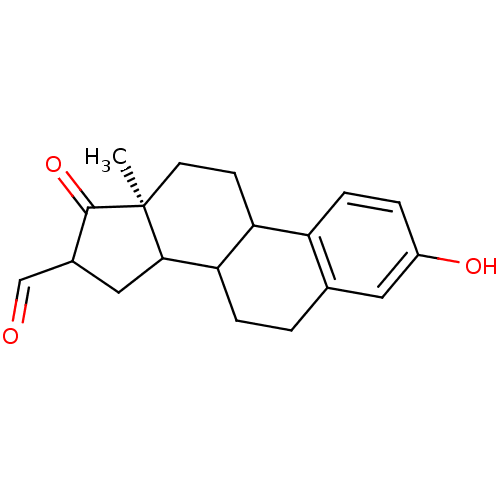
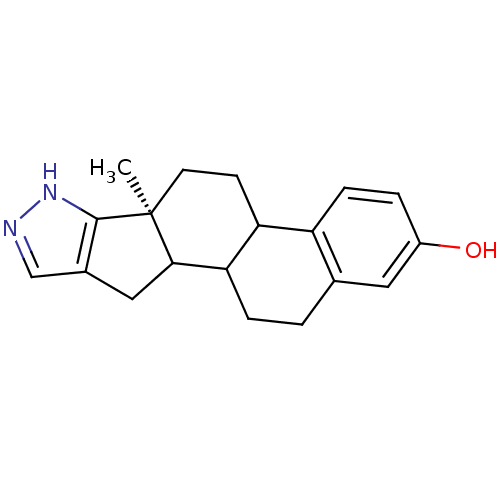
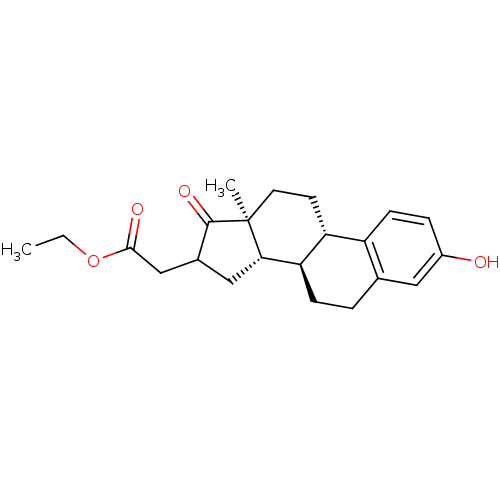
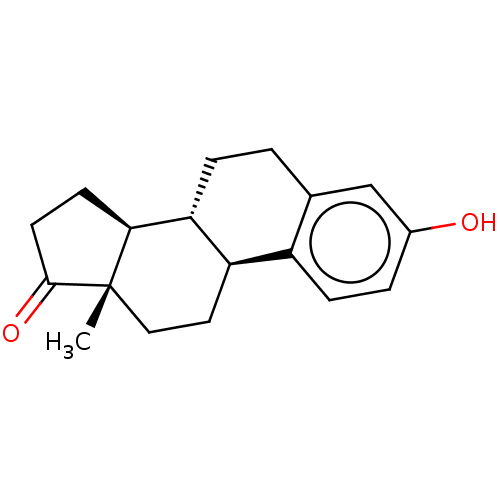
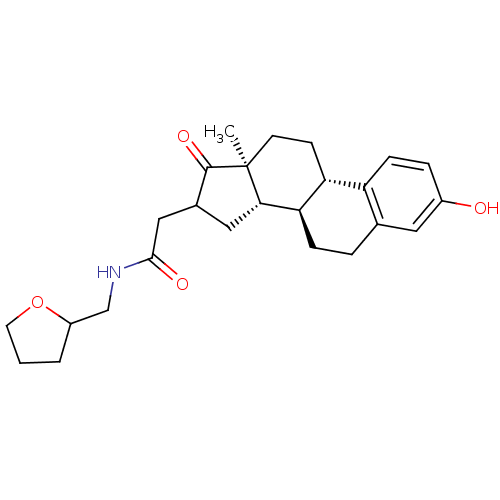
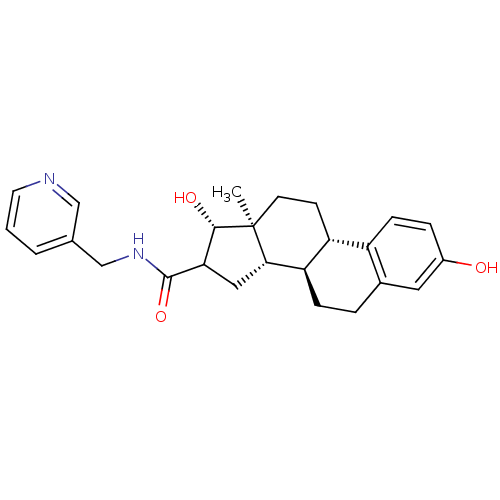
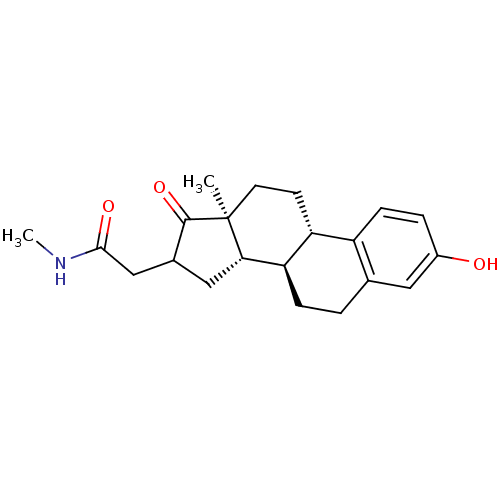
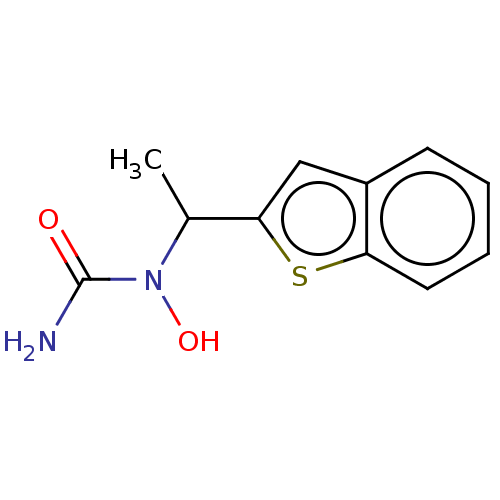
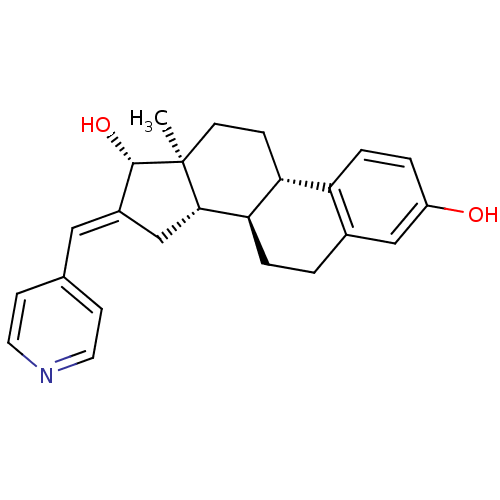
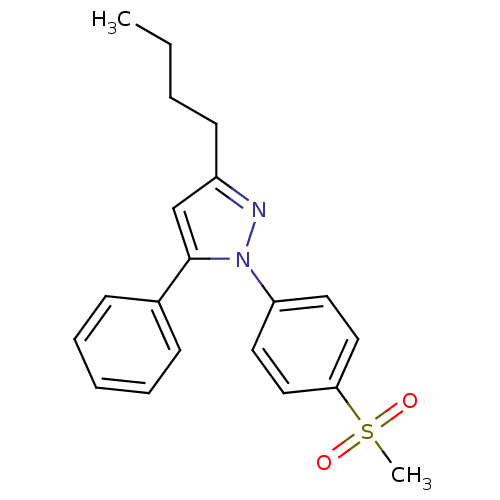
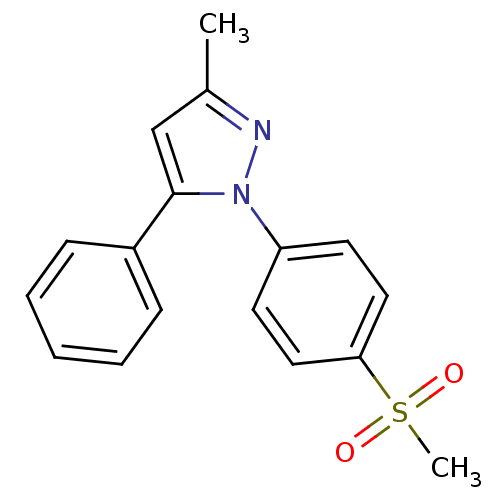
Affinity DataIC50: 1nMAssay Description:The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2More data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 37nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2More data for this Ligand-Target Pair
Affinity DataIC50: <100nMAssay Description:The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 2More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 110nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 150nMAssay Description:Inhibition of 5-lipoxygenase activity of compound evaluated as determined by the inhibition of calcium ionophore-induced leukotriene B4 production in...More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 300nMAssay Description:The 5-lipoxygenase activity of the compound was determined by the inhibition of calcium ionophore-induced leukotriene B4 production in human blood.More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 320nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 330nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 340nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 380nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 460nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 510nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 810nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 1.10E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 1More data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 2.90E+3nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Target17-beta-hydroxysteroid dehydrogenase type 1(Homo sapiens (Human))
University Of Bath
Curated by ChEMBL
University Of Bath
Curated by ChEMBL
Affinity DataIC50: 4.10E+3nMAssay Description:Inhibition of 17-beta HSD1 in T47D cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 6.00E+3nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 2 using osteosarcomes cellsMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against Prostaglandin G/H synthase 1 using monocytes-like cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 3.80E+4nMAssay Description:The compound was evaluated for prostaglandin E2 inhibition using recombinant Prostaglandin G/H synthase 1More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid 5-lipoxygenase(Homo sapiens (Human))
Laboratoires Innothera
Curated by ChEMBL
Laboratoires Innothera
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:The compound was evaluated for its inhibitory activity against 5-lipoxygenase using granulocytes-type cellsMore data for this Ligand-Target Pair

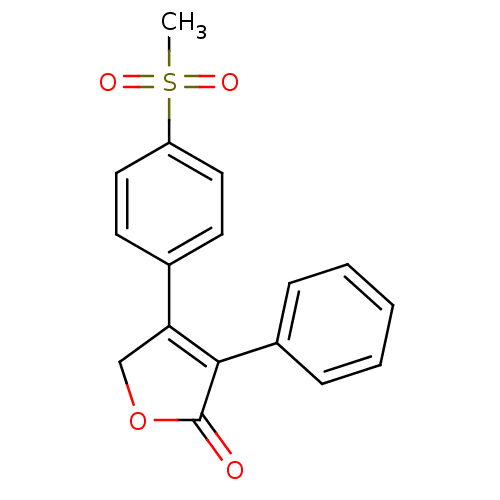
 3D Structure (crystal)
3D Structure (crystal)