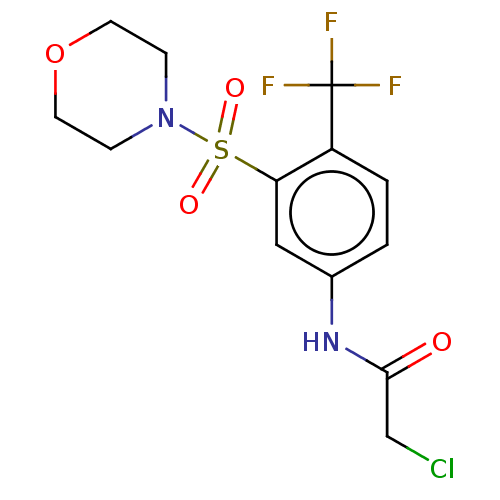
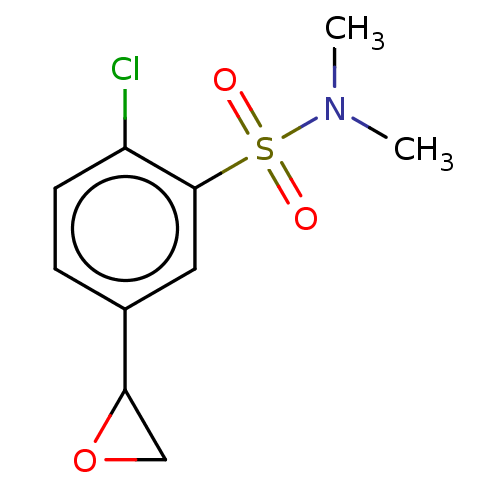
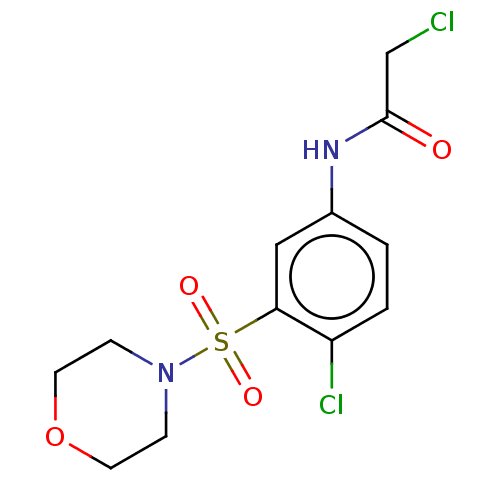
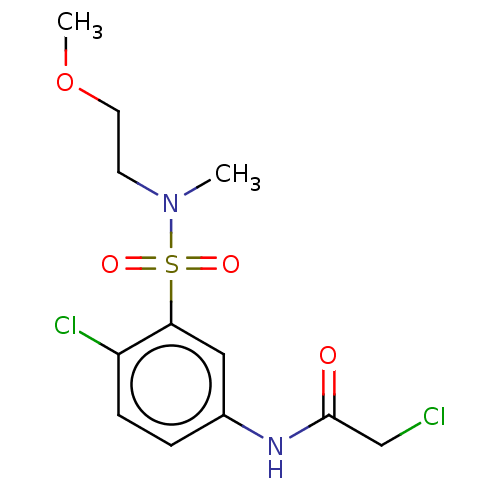
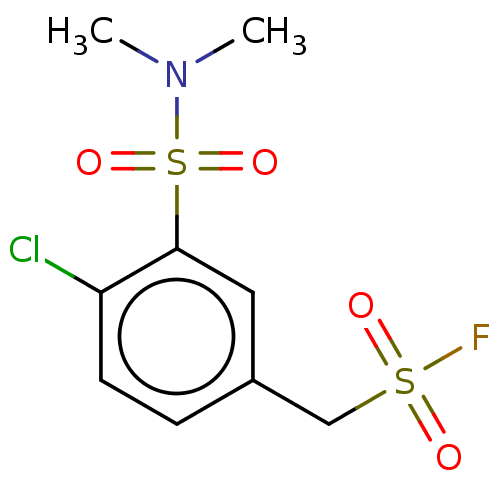
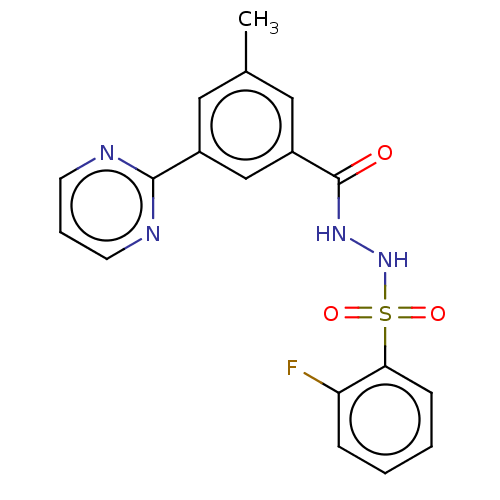
Affinity DataKi: 36nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
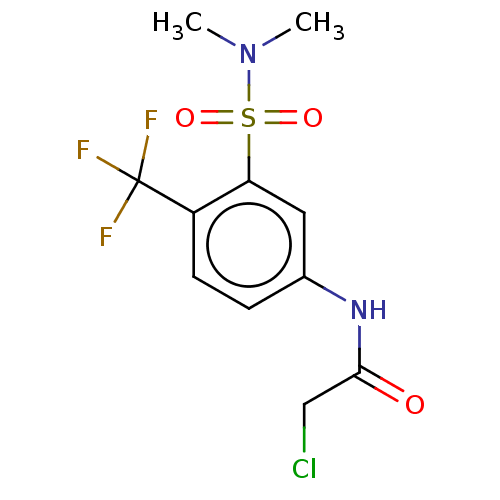
Affinity DataKi: 41nMAssay Description:Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
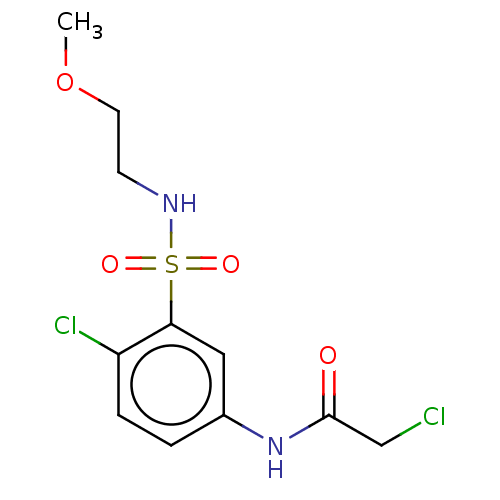
Affinity DataKi: 160nMAssay Description:Competitive inhibition of human recombinant CYP1A1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
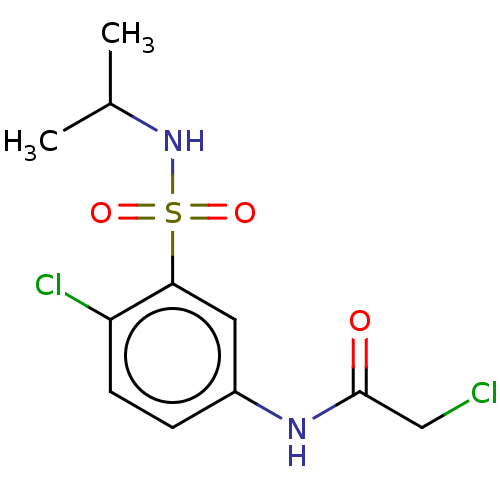
Affinity DataKi: 190nMAssay Description:Competitive inhibition of human recombinant CYP3A5 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 203nMAssay Description:Displacement of [3H]-HU-243 from CB2 receptor (unknown origin) expressed in African green monkey Cos7 cell membranes incubated for 90 mins by radioli...More data for this Ligand-Target Pair
Affinity DataKi: 440nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 610nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Mixed type inhibition of human recombinant CYP2B6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 790nMAssay Description:Mixed type inhibition of human recombinant CYP2C19 using (S)-mephenytoin as substrate preincubated for 5 mins followed by NADPH-generating system add...More data for this Ligand-Target Pair
Affinity DataKi: 842nMAssay Description:Displacement of [3H]-HU-243 from CB1 receptor in Sabra rat brain synaptosomes incubated for 90 mins by radioligand binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+3nMAssay Description:Competitive inhibition of human recombinant CYP3A4 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 15 mins ...More data for this Ligand-Target Pair
Affinity DataKi: 1.10E+3nMAssay Description:Inhibition of 5-HT2C (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30E+3nMAssay Description:Inhibition of mu-type opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.30E+3nMAssay Description:Inhibition of kappa-type opioid receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.42E+3nMAssay Description:Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 10 mins in presence of NADPH generating by Lineweave...More data for this Ligand-Target Pair
Affinity DataKi: 2.50E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 2.69E+3nMAssay Description:Competitive inhibition of human recombinant CYP1A2 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.70E+3nMAssay Description:Inhibition of D1 dopamine receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 2.86E+3nMAssay Description:Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 3.10E+3nMAssay Description:Inhibition of histamine H3 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of alpha2B receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.40E+3nMAssay Description:Inhibition of sigma2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 3.63E+3nMAssay Description:Competitive inhibition of human recombinant CYP1B1 using 7-Ethoxyresorufin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 3.70E+3nMAssay Description:Inhibition of alpha2C receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 4.35E+3nMAssay Description:Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometryMore data for this Ligand-Target Pair
Affinity DataKi: 4.80E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 5.00E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+3nMAssay Description:Competitive inhibition of CYP2C9 in pooled human liver microsomes using S-warfarin as substrate preincubated for 5 mins followed by NADPH-generating ...More data for this Ligand-Target Pair
Affinity DataKi: 6.40E+3nMAssay Description:Inhibition of delta-type opioid receptor receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 6.70E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 6.90E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 9.60E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 9.90E+3nMAssay Description:Inhibition of recombinant GSTO1-1 (unknown origin) expressed in Escherichia coli assessed as inhibitor constant using S-(4-nitrophenacyl)glutathione ...More data for this Ligand-Target Pair
Affinity DataKi: 1.23E+4nMAssay Description:Mixed type inhibition of human recombinant CYP3A7 expressed in baculovirus-infected insect cells using diltiazem as substrate incubated for 30 mins f...More data for this Ligand-Target Pair
Affinity DataKi: 2.07E+4nMAssay Description:Competitive inhibition of CYP2C11 in rat liver microsomesMore data for this Ligand-Target Pair
Affinity DataKi: 5.50E+4nMAssay Description:Non-competitive inhibition of human recombinant CYP2A6 expressed in baculovirus-infected insect cells using coumarin as substrate preincubated for 5 ...More data for this Ligand-Target Pair
TargetSteroid 17-alpha-hydroxylase/17,20 lyase(Rattus norvegicus (Rat))
University Of Minnesota
Curated by ChEMBL
University Of Minnesota
Curated by ChEMBL
Affinity DataKi: 1.24E+5nMAssay Description:Mixed type inhibition of CYP17 in Sprague-Dawley rat testis microsomes assessed as reduction in 17alpha-Hydroxylase activity in presence of NADPH-gen...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 5.5nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 6.30nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 7nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 8nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 9nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetHistone acetyltransferase KAT6A [507-778](Homo sapiens)
Cancer Therapeutics Crc
Curated by ChEMBL
Cancer Therapeutics Crc
Curated by ChEMBL
Affinity DataIC50: 9.40nMAssay Description:Inhibition of N-terminal NusA-fused His6-tagged human KAT6A (507 to 778 residues) expressed in Escherichia coli BL21 (DE3) cells using 0.4 uM acetyl ...More data for this Ligand-Target Pair
TargetA5LHX3/O14818/P20618/P25786/P25787/P25788/P25789/P28062/P28065/P28066/P28070/P28072/P28074/P40306/P49720/P49721/P60900/Q8TAA3/Q99436(Homo sapiens (Human))
Monash University
Curated by ChEMBL
Monash University
Curated by ChEMBL
Affinity DataIC50: <10nMAssay Description:Inhibition of 20S proteasome activity in human ANBL-6 cellsMore data for this Ligand-Target Pair

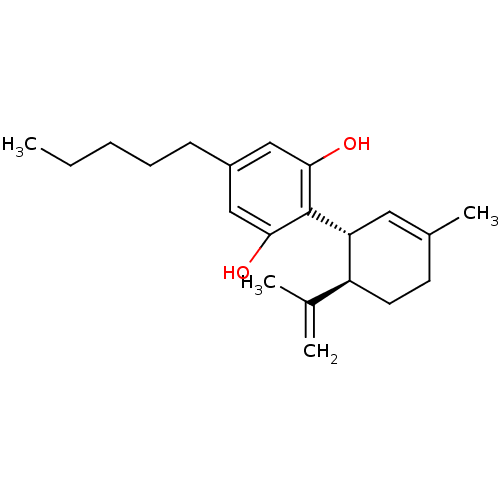
 3D Structure (crystal)
3D Structure (crystal)