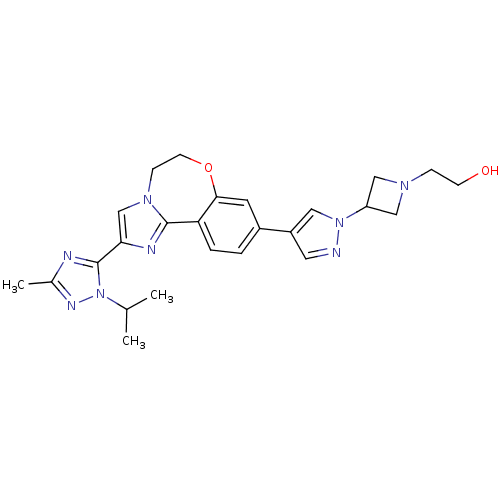
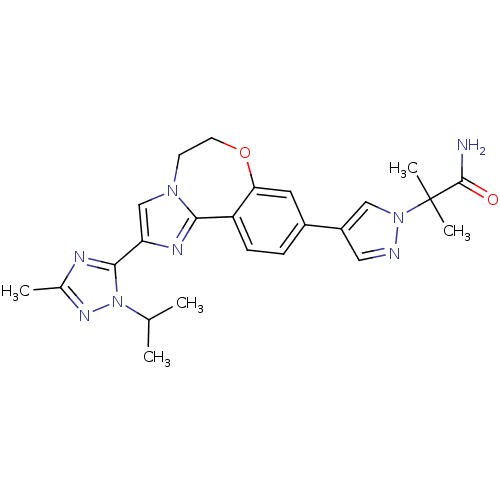
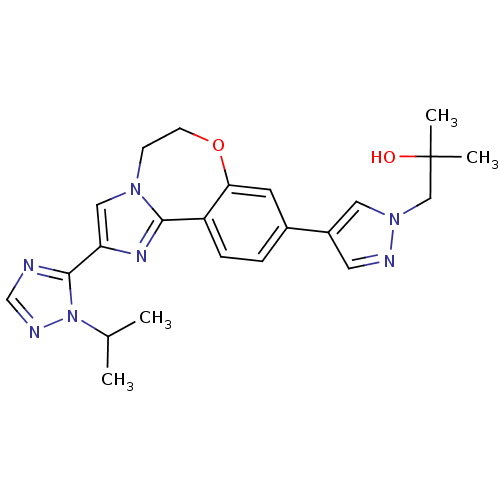
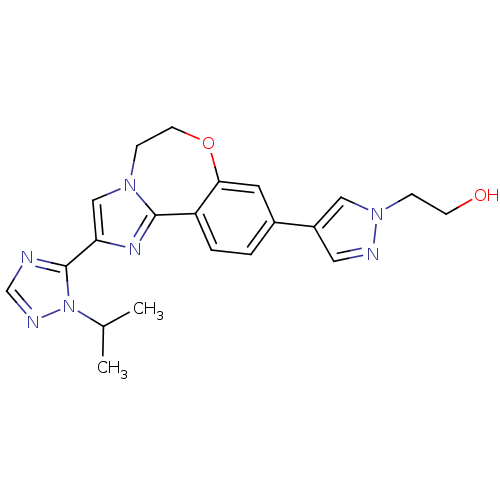
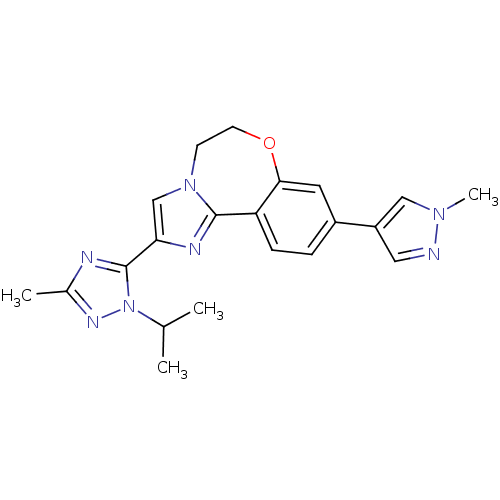
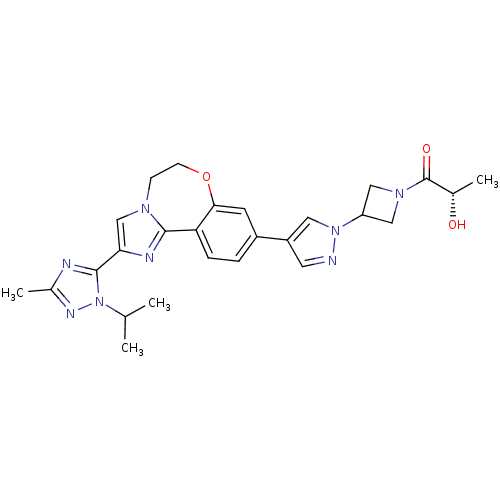
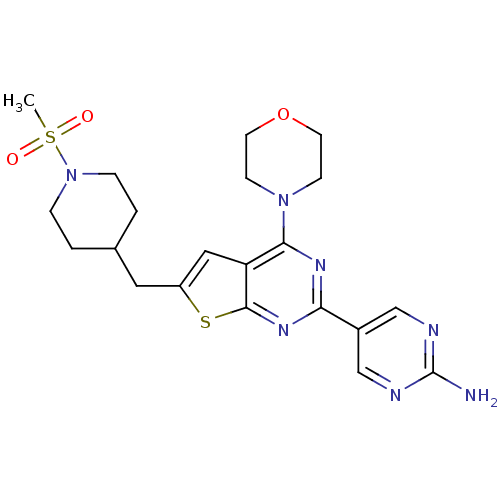
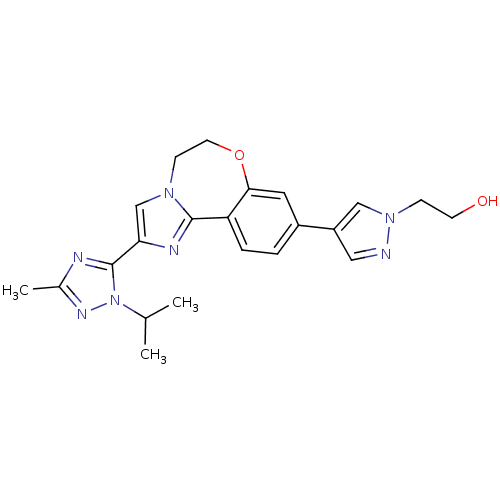
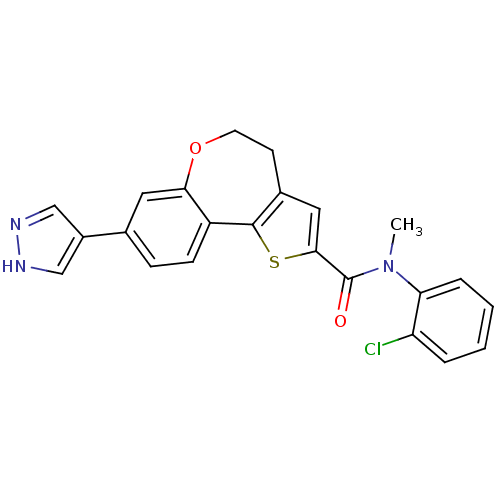
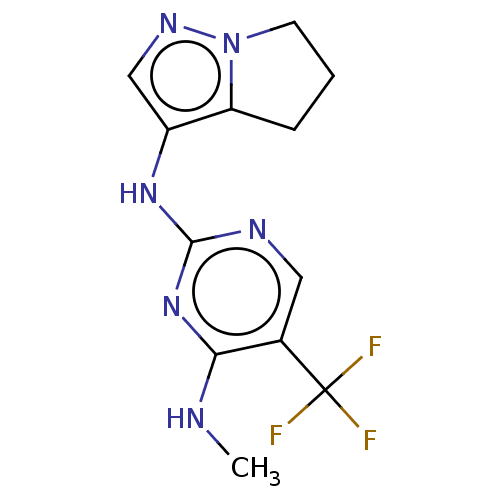
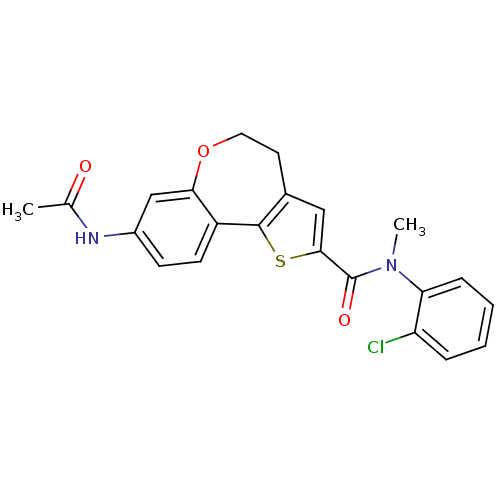
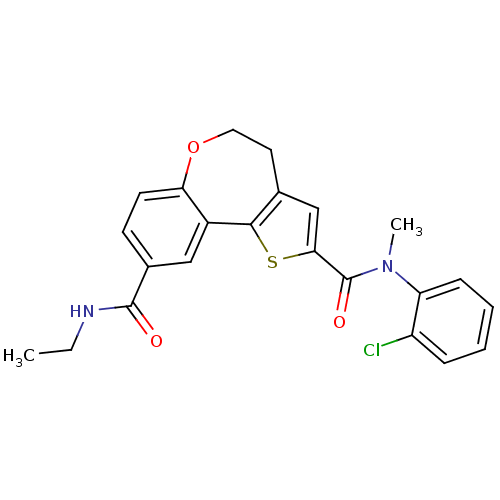
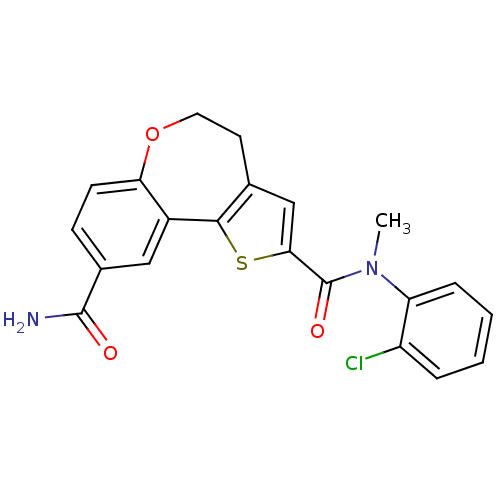
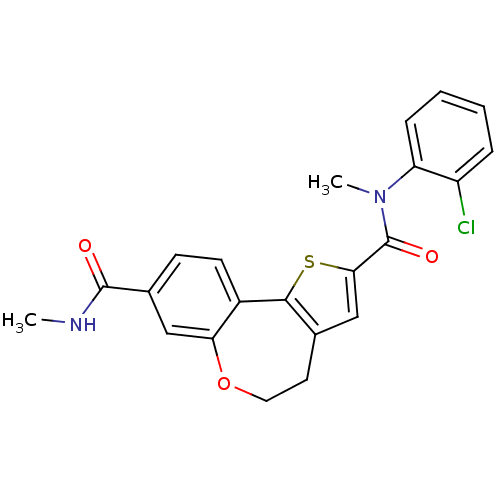
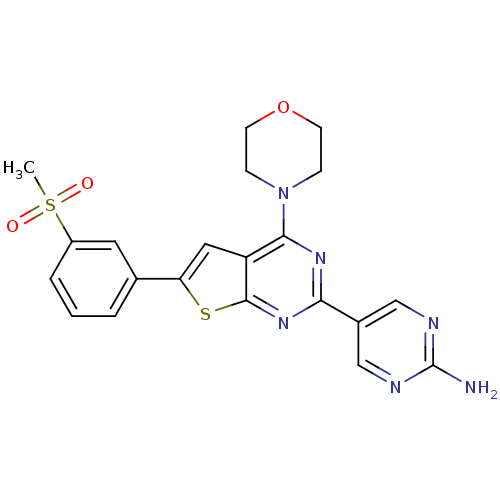
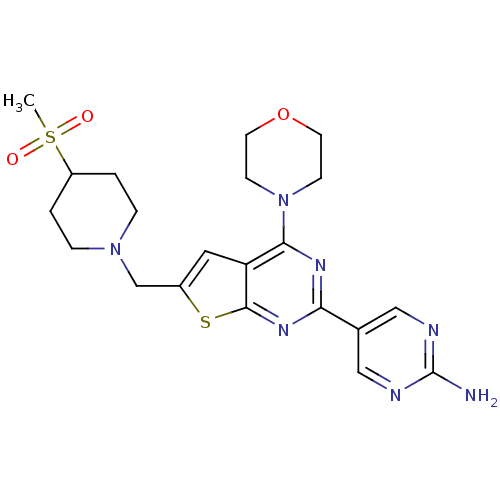
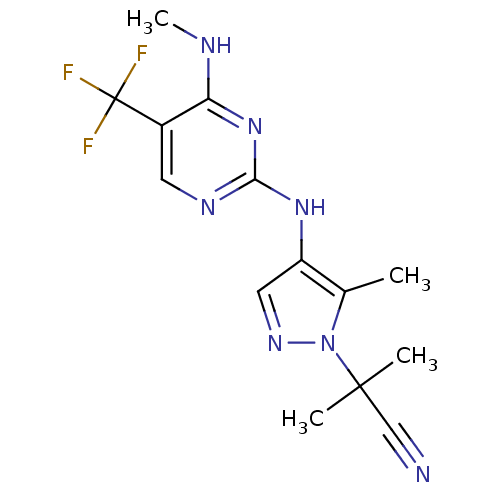
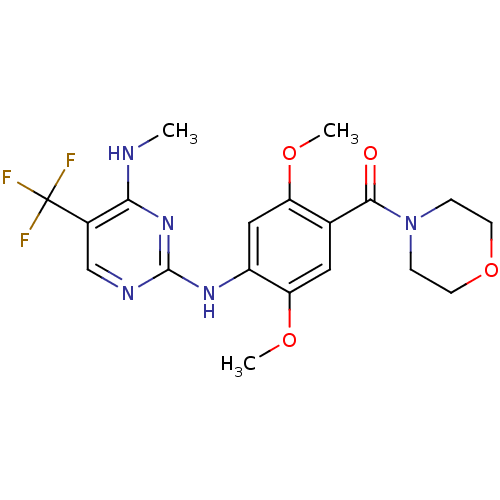
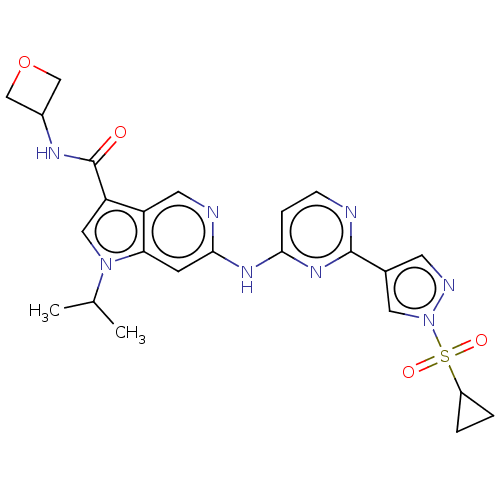
Affinity DataKi: 0.100nMAssay Description:Inhibition of wild-type EGFR (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
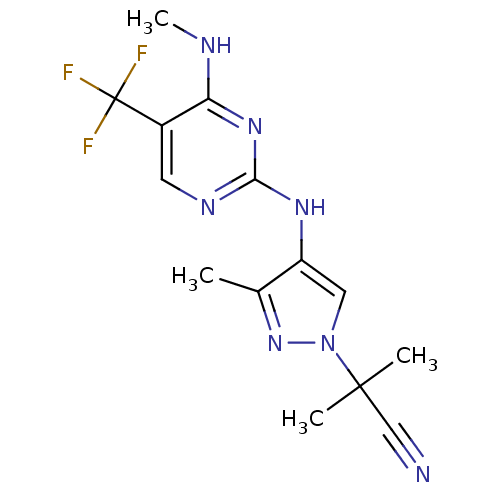
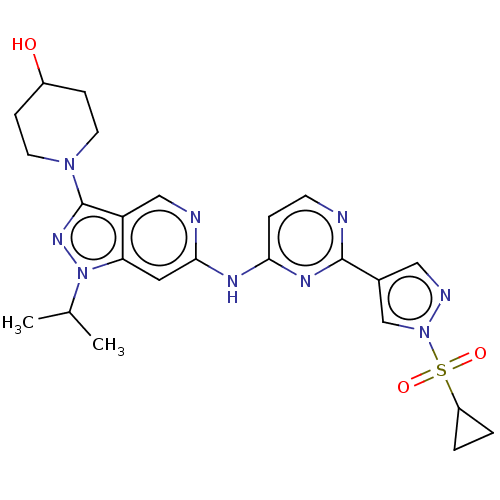
Affinity DataKi: 0.100nMAssay Description:Inhibition of EGFR T790M/L858R mutant (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
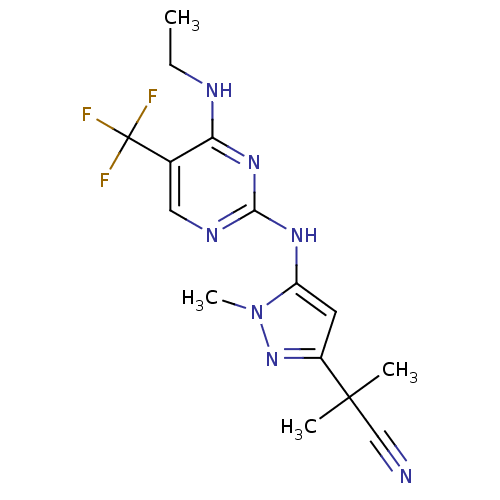
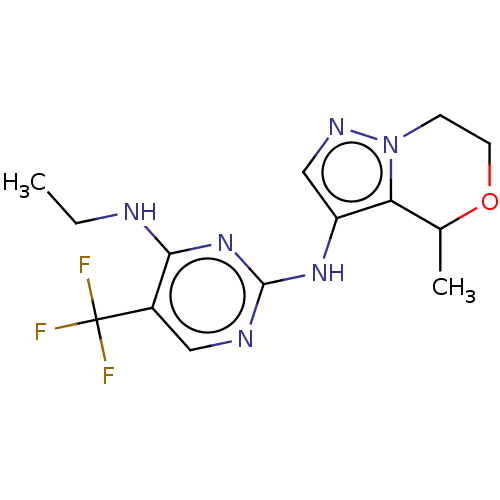
Affinity DataKi: 0.100nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass...More data for this Ligand-Target Pair
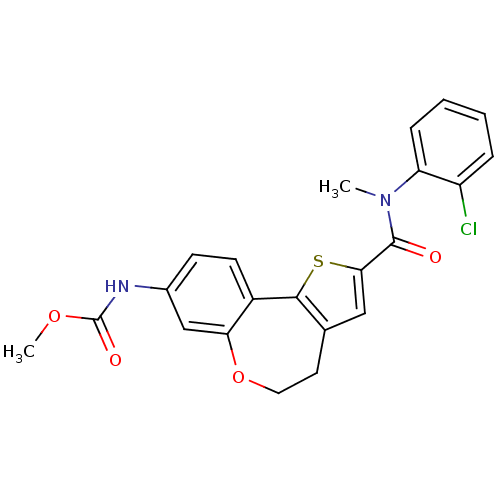
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
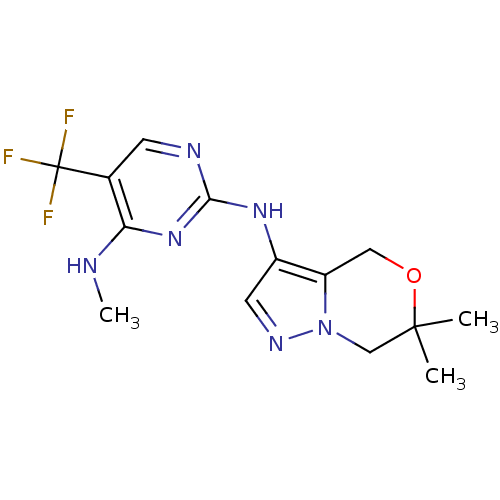
Affinity DataKi: 0.110nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.120nMAssay Description:Inhibition of PI3Kdelta (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.140nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.170nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.290nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
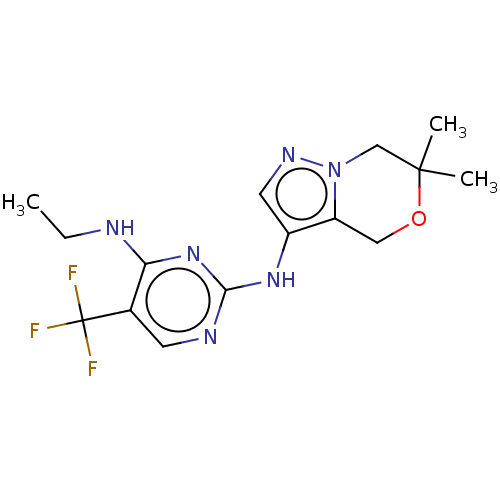
Affinity DataKi: 0.300nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.300nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Inhibition of EGFR T790M/del746 to 750 mutant (unknown origin) by high-throughput biochemical screeningMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.310nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.350nMAssay Description:Inhibition of human EGFR T790M/del (746 to 750 residues) mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 peptide as subs...More data for this Ligand-Target Pair
Ligand Info
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.400nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.600nMAssay Description:Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
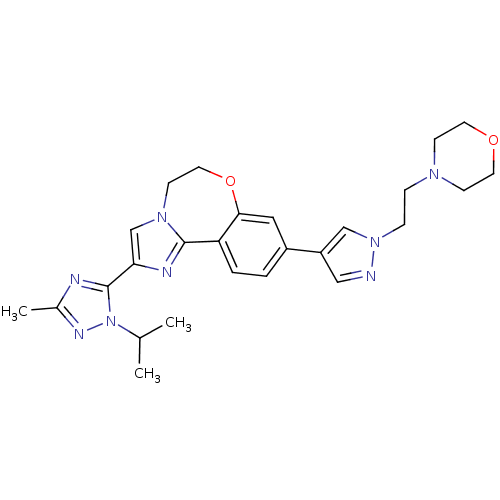
Affinity DataKi: 0.600nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: <0.600nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: <0.600nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
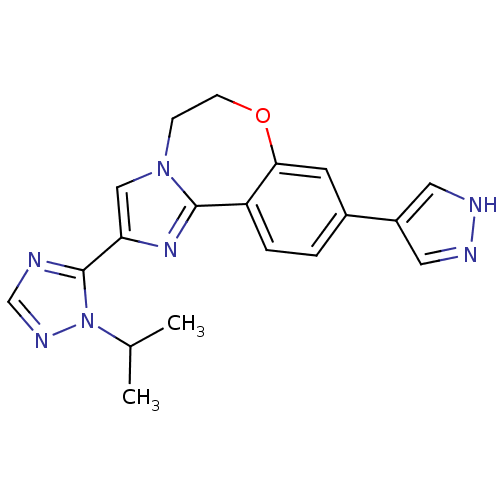
Affinity DataKi: 0.700nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.700nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 0.790nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 0.900nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
Affinity DataKi: 0.900nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 0.970nMAssay Description:Inhibition of PI3Kgamma (unknown origin)More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assayMore data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of LRRK2 using FAM-LRRKtide as substrate after 120 mins by microfluidic capillary electrophoresis assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: <1nMAssay Description:Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.10nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.20nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand InfoPDB
Affinity DataKi: 1.20nMAssay Description:Inhibition of human EGFR T790M/L858R double mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as peptide substrate preincu...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.20nMAssay Description:This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of human EGFR del (746 to 750) mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as substrate preincubated for ...More data for this Ligand-Target Pair
Ligand Info
Affinity DataKi: 1.40nMAssay Description:Inhibition of human EGFR del (746 to 750) mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 as substrate preincubated for ...More data for this Ligand-Target Pair
Ligand InfoPDB
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataKi: 1.5nMAssay Description:Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of human EGFR T790M/del (746 to 750 residues) mutant assessed as apparent inhibition constant using Fl-EEPLYWSFPAKKK-CONH2 peptide as subs...More data for this Ligand-Target Pair
Ligand InfoPDB

 3D Structure (crystal)
3D Structure (crystal)