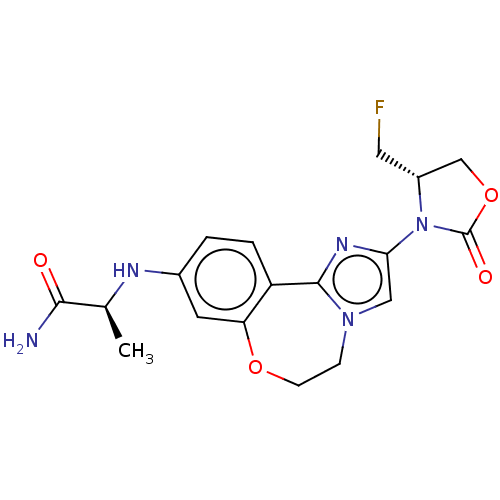
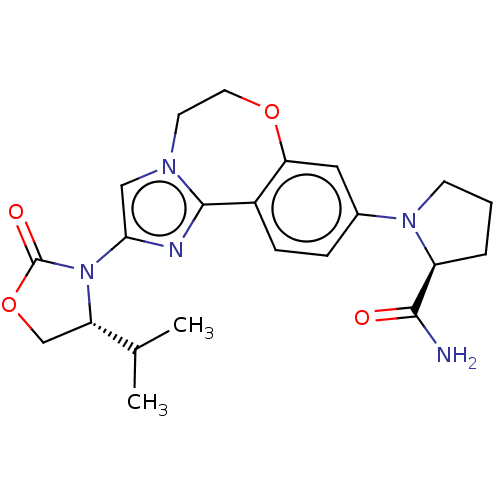
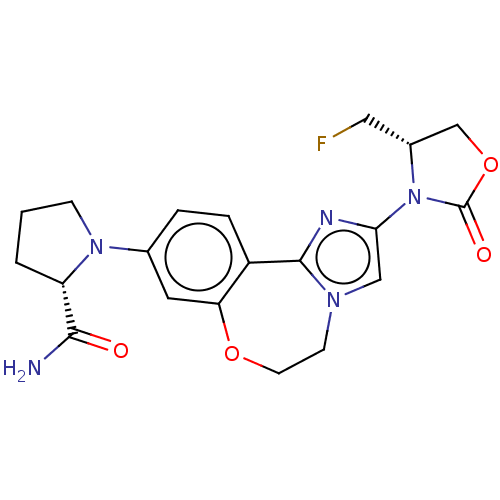
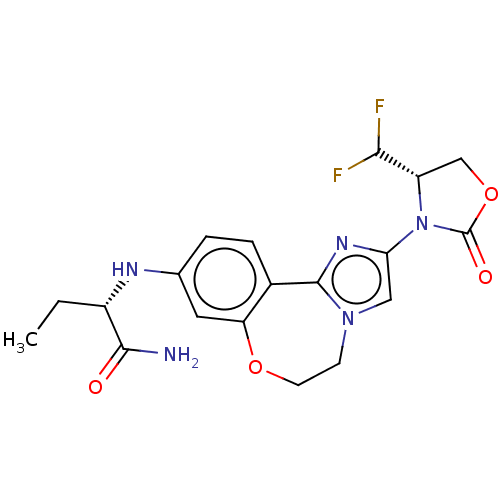
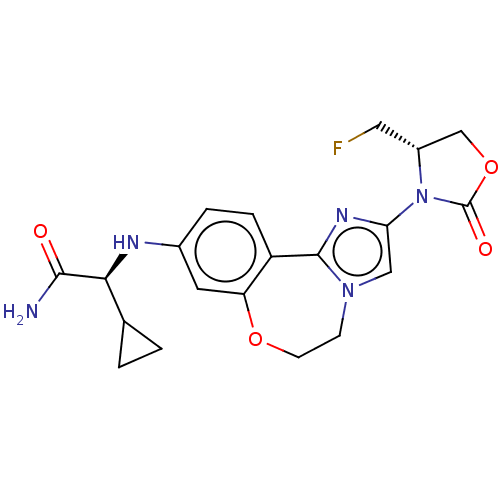
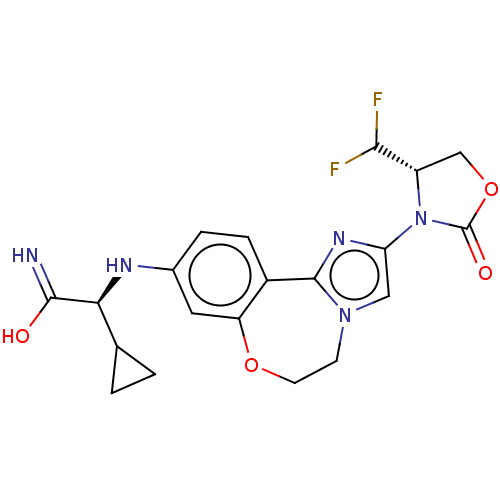
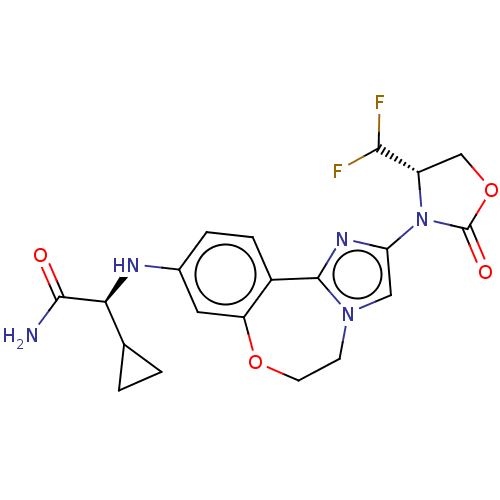
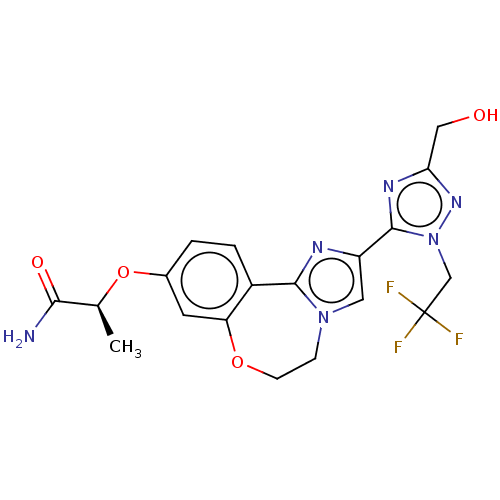
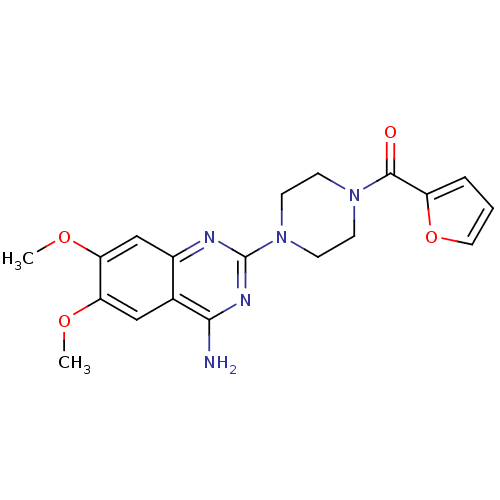
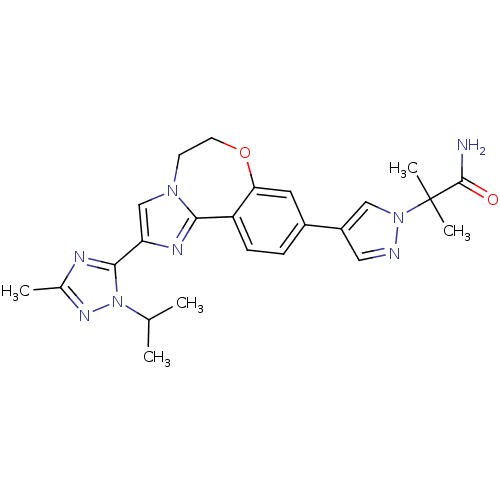
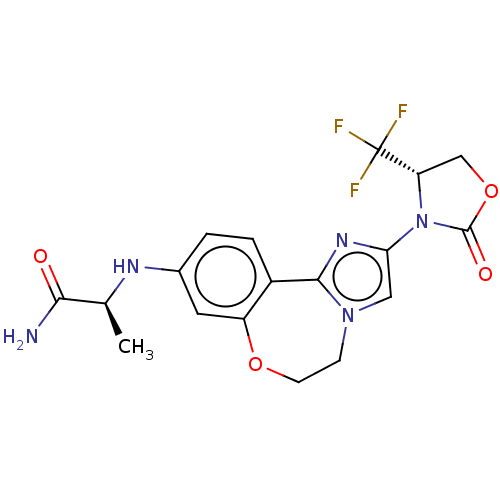
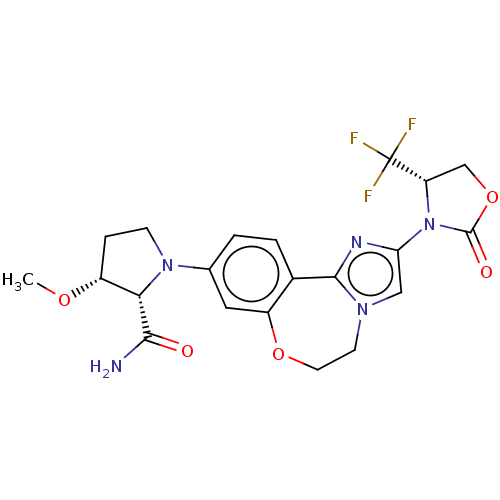
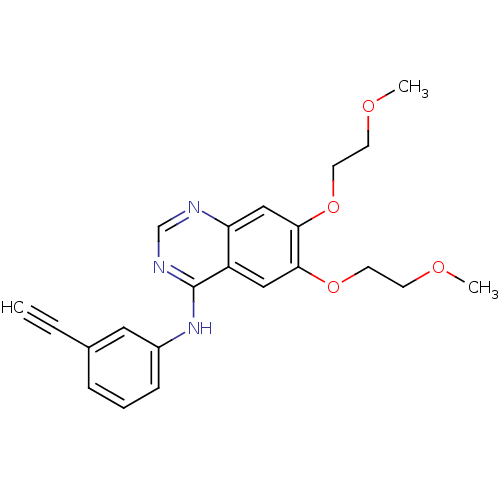
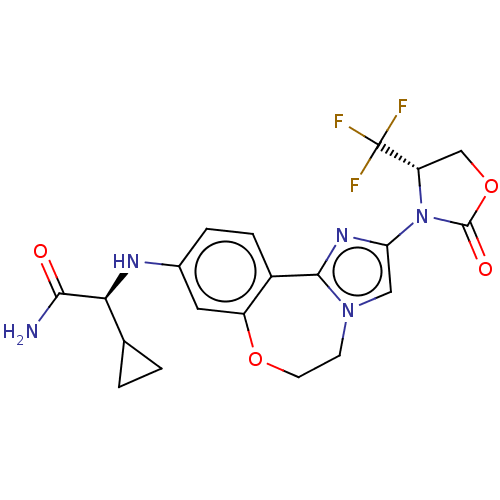
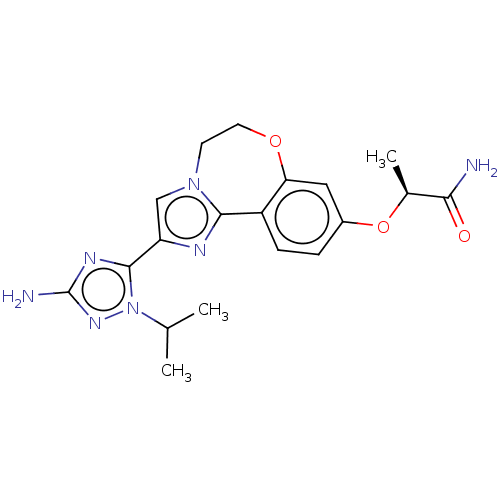
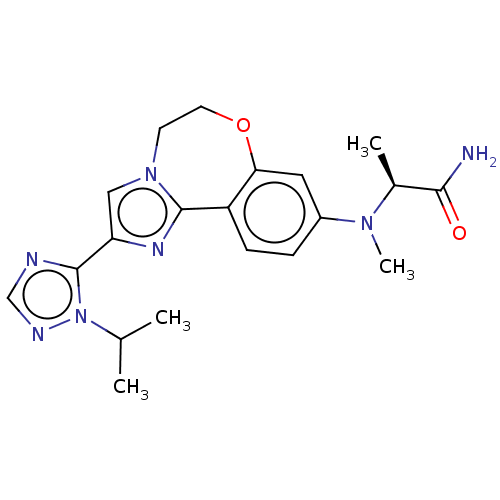
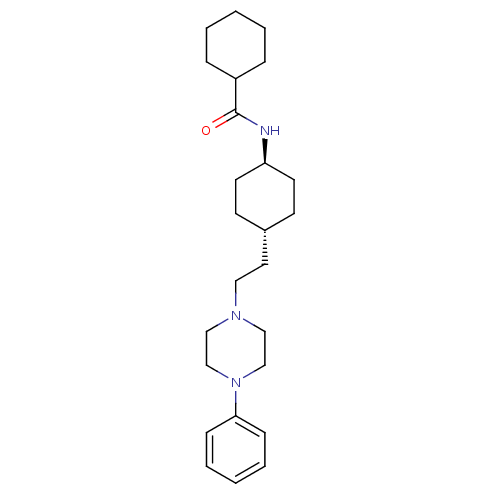
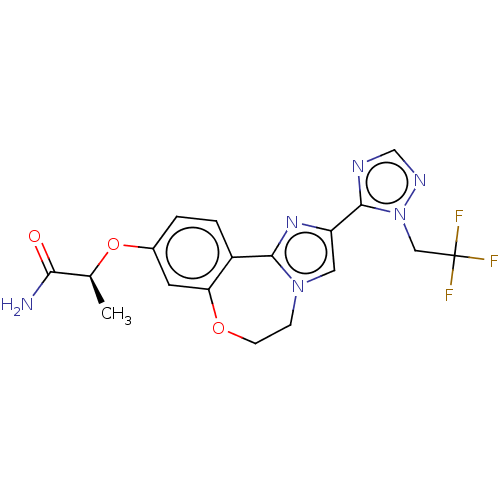
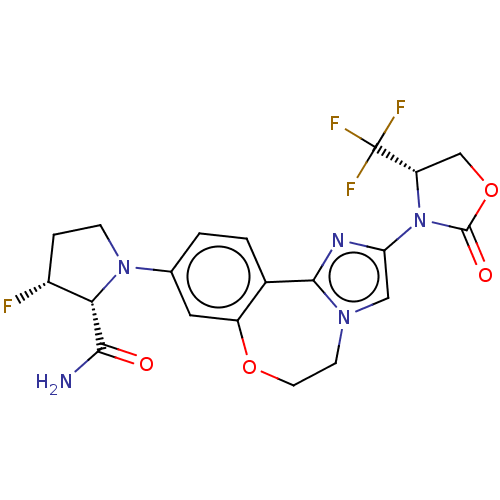
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0160nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0160nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMAssay Description:Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes.More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: <0.0200nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: <0.0200nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0230nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0230nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0260nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPlatelet-activating factor acetylhydrolase(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataKi: 0.0300nMAssay Description:Steady state and transient kinetics to a freely reversible, non-covalently bound, human recombinant Phospholipase A2 (rhLp-PLA2) was determinedMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0340nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0400nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0400nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0420nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0430nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0480nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0480nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0510nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0510nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0510nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0530nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0600nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0600nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0600nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0620nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0700nMAssay Description:Inhibition of [3H]- prazosin binding against Alpha-1A adrenergic receptor from rat submaxillary glandMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0790nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0790nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0790nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0900nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0900nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0900nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0900nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0950nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.0970nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.100nMAssay Description:Inhibition of recombinant human N-terminal GST-tagged EGFR catalytic domain (669 to 1210 residues) expressed in baculovirus expression system by mass...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.100nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.107nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.107nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.107nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes.More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.150nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Inhibition of [3H]- prazosin binding against Alpha-1B adrenergic receptor from rat liverMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.157nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 3-kinase regulatory subunit alpha(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.186nMAssay Description:PI3K Binding assays are intended for determining the biochemical potency of small molecule PI3K inhibitors. The PI3K lipid kinase reaction is perform...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.186nMAssay Description:The biochemical inhibition of four PI3K isoforms by the Formula I compounds of Table 1. In addition, two clinically tested PI3K compounds, taselisib ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.188nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform(Homo sapiens (Human))
Genentech
US Patent
Genentech
US Patent
Affinity DataKi: 0.289nMAssay Description:Inhibition of PI3Kalpha (unknown origin) using PIP2:3PS as substrate in presence of ATP measured after 120 mins by ADP-Glo assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Binding affinity towards human neuropeptide Y receptor type 5 using 125[I]-PYY as radioligandMore data for this Ligand-Target Pair

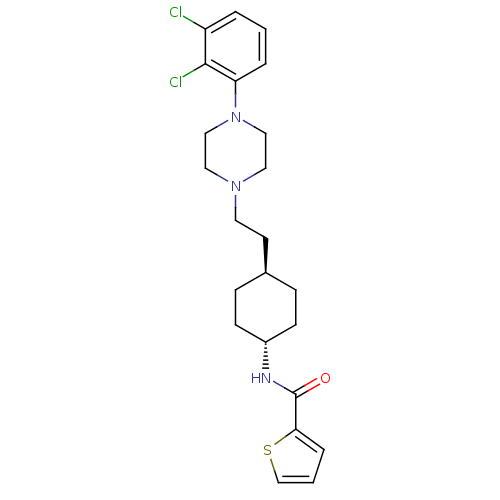
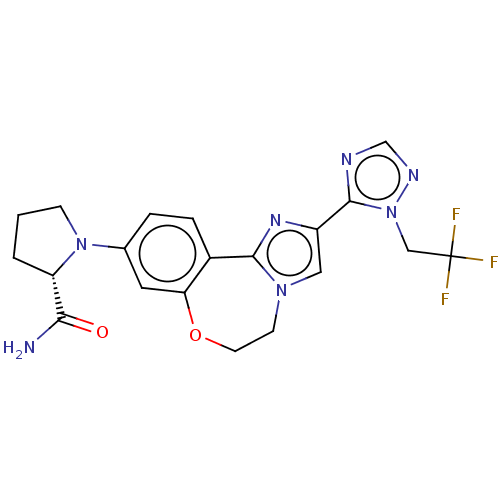
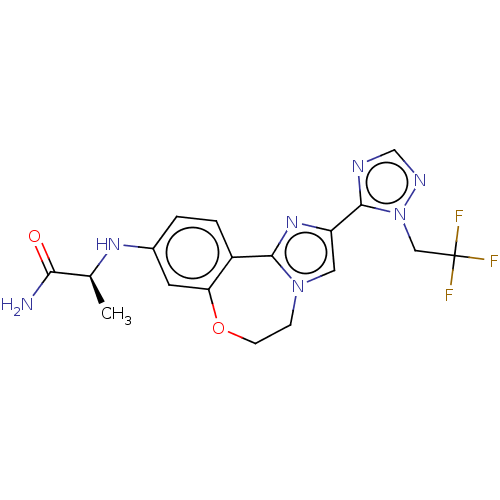
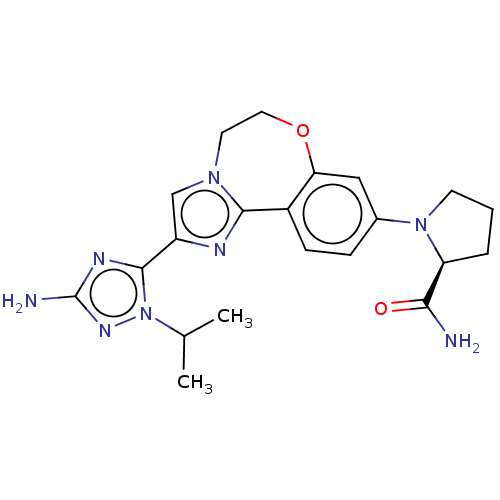
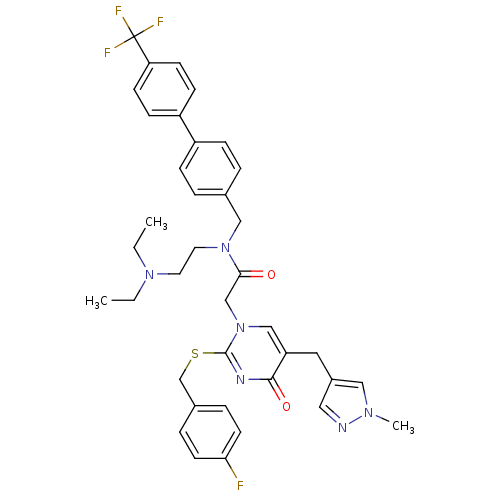
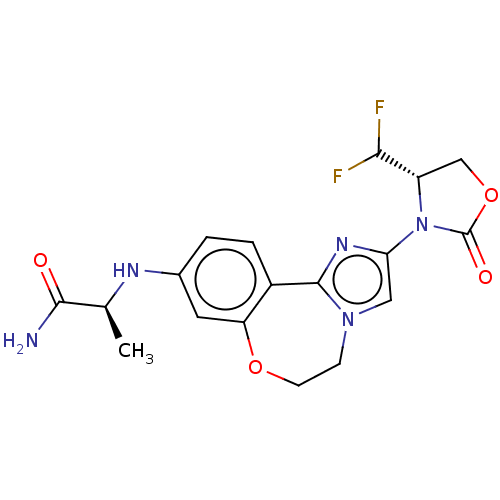
 3D Structure (crystal)
3D Structure (crystal)