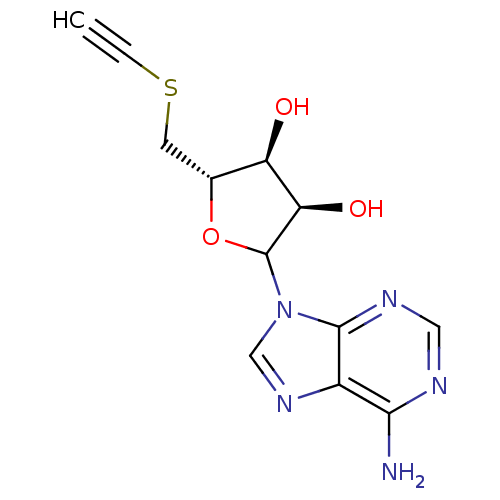
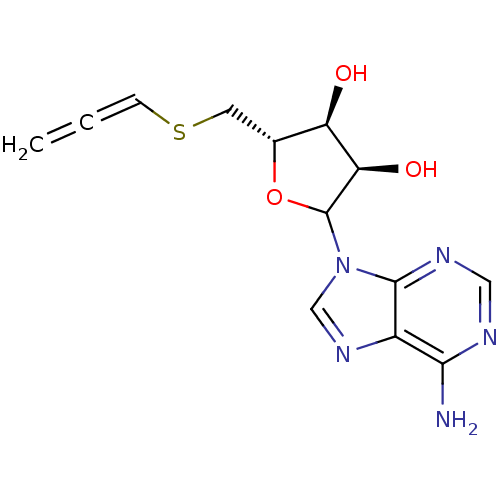
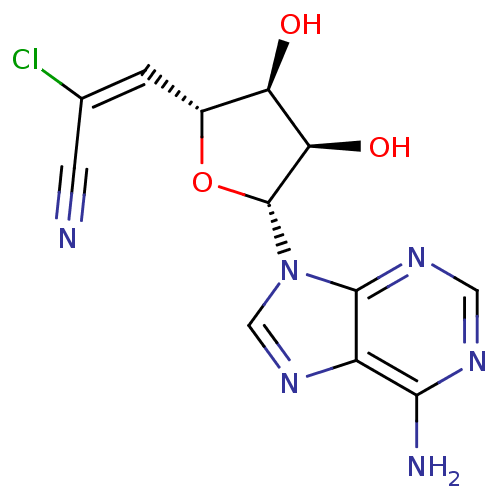
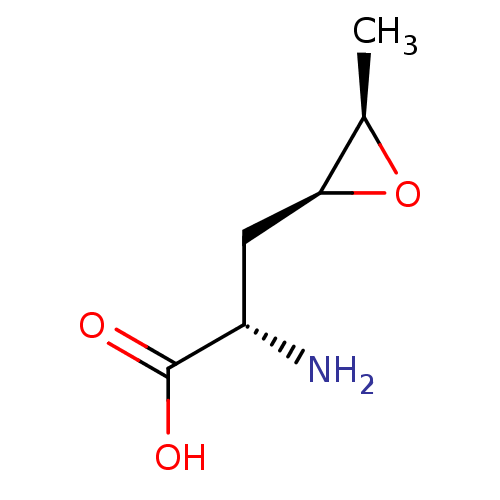
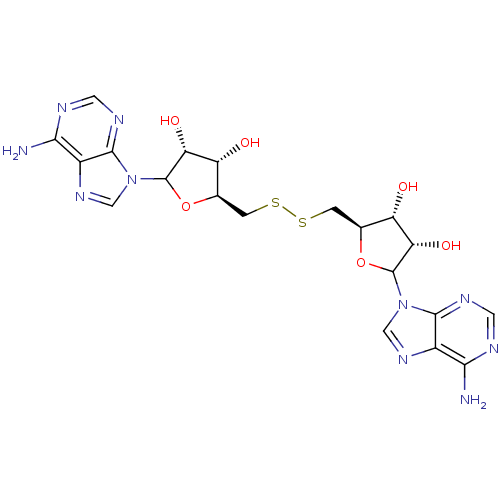
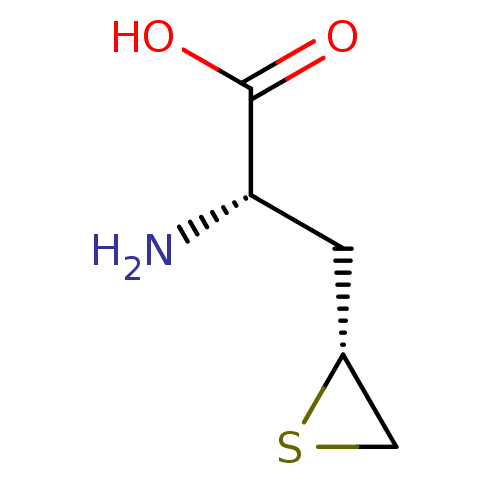
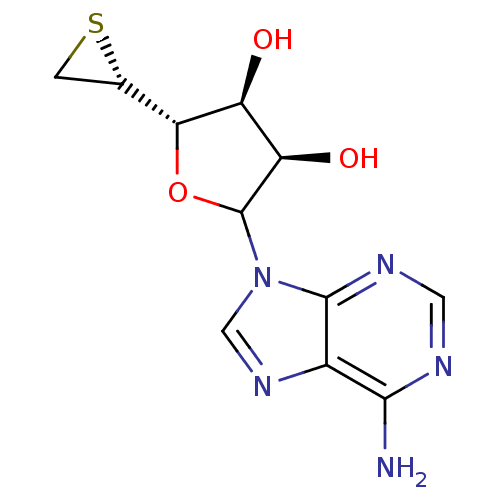
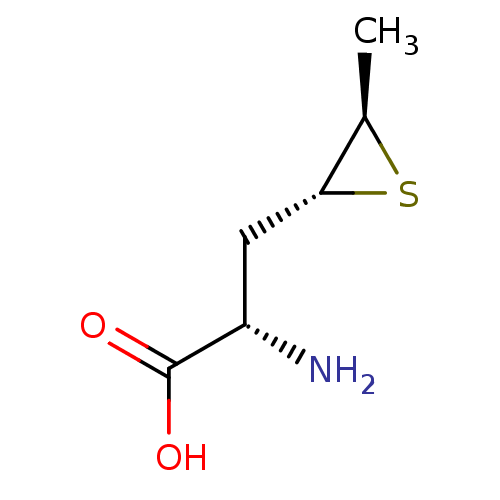
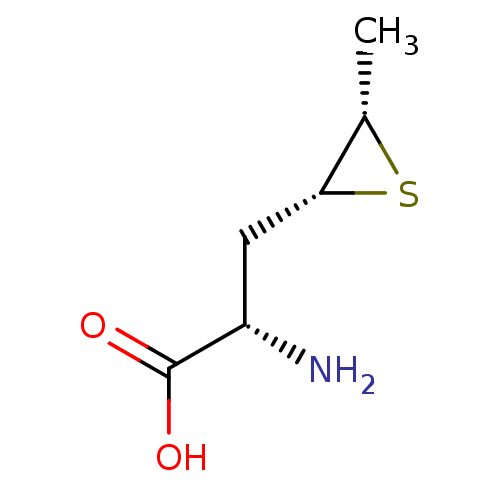
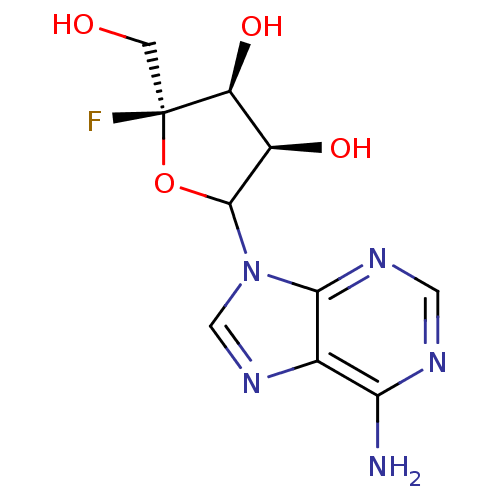
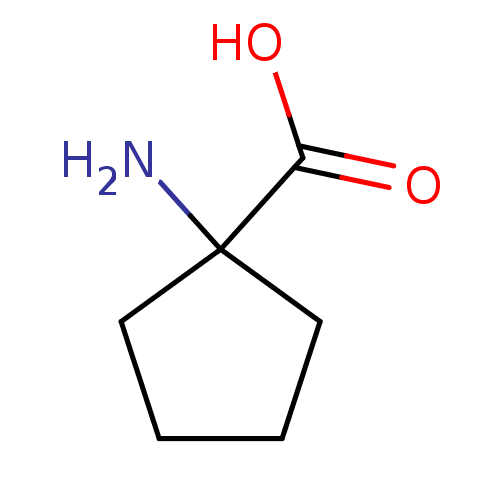
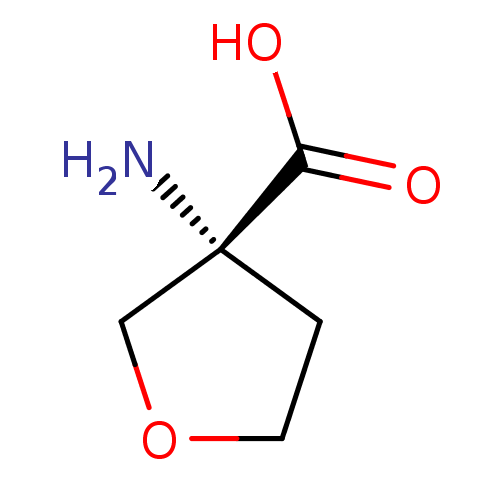
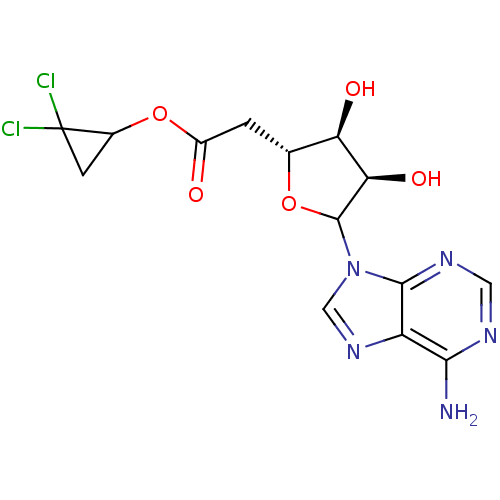
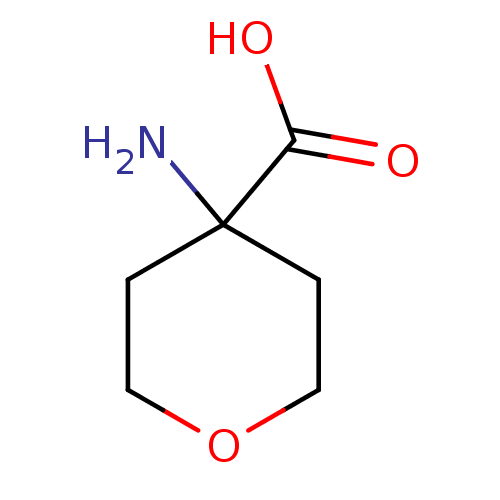
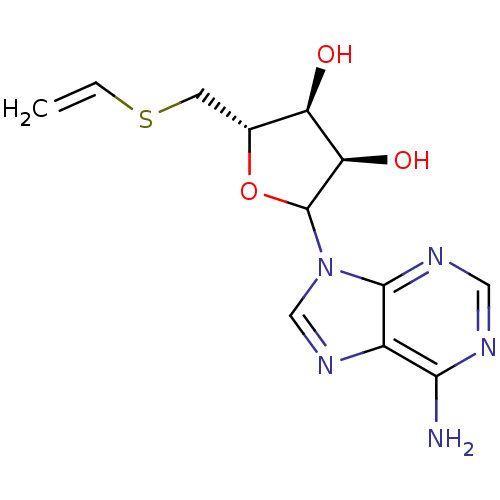
Affinity DataKi: 3.20E+3nMAssay Description:Inhibition of human placental AdoHcy hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity for human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.00E+3nMAssay Description:Binding affinity against S-Adenosyl-homocysteine hydrolase was determinedMore data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
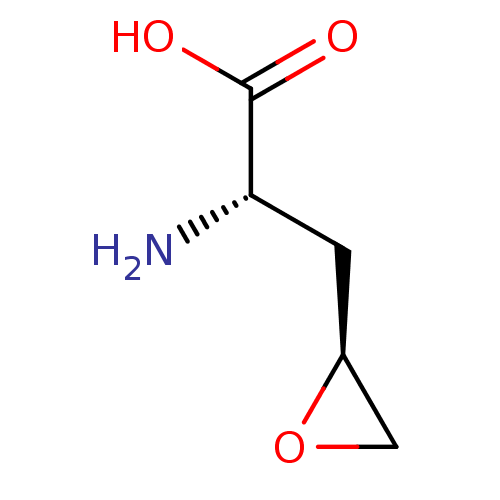
Affinity DataKi: 7.00E+3nM ΔG°: -30.6kJ/mole IC50: 5.00E+4nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+4nMAssay Description:Binding affinity against S-Adenosyl-homocysteine hydrolase was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of purified recombinant human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nMAssay Description:Inhibition of purified recombinant human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Inhibition of human placental AdoHcy hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.70E+4nMAssay Description:Inhibitory activity against AdoHcy HydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.90E+4nMAssay Description:Binding affinity for human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 4.50E+4nMAssay Description:Inhibition of purified recombinant human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.00E+4nMAssay Description:Binding affinity for human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.60E+4nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
Affinity DataKi: 6.10E+4nM ΔG°: -25.0kJ/mole IC50: 1.00E+5nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
Affinity DataKi: 6.70E+4nMAssay Description:Binding affinity against S-Adenosyl-homocysteine hydrolase was determinedMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+4nMAssay Description:Inhibition of human placental AdoHcy hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 9.60E+4nMAssay Description:Binding affinity for human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
Affinity DataKi: 1.00E+5nM ΔG°: -23.7kJ/mole IC50: 1.40E+5nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
Affinity DataKi: 1.05E+5nM ΔG°: -23.6kJ/mole IC50: 1.10E+5nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
Affinity DataKi: 1.05E+5nMAssay Description:Binding affinity for human placental S-adenosyl-homocysteine hydrolaseMore data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
Affinity DataKi: 1.40E+5nM ΔG°: -22.9kJ/mole IC50: 3.00E+5nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
TargetS-adenosylmethionine synthase isoform type-2(Rattus norvegicus)
Laboratoire De Chimie Bioorganique
Laboratoire De Chimie Bioorganique
Affinity DataKi: 1.40E+5nM ΔG°: -22.9kJ/mole IC50: 4.00E+5nMpH: 7.5 T: 2°CAssay Description:AdoMet synthetase activity was measured by a radioactive assay using (14C-methyl)-L-methionine as described by Sullivan and Hoffman with minor modifi...More data for this Ligand-Target Pair
Affinity DataKi: 1.66E+5nMAssay Description:Inhibitory constant against rat liver S-adenosyl-L-homocysteine hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 5.16E+5nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+5nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 7.50E+5nMAssay Description:Inhibition of human placental AdoHcy hydrolaseMore data for this Ligand-Target Pair
Affinity DataKi: 1.00E+6nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 1.04E+6nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair
Affinity DataKi: 3.00E+6nMAssay Description:Compound was evaluated for its inhibitory activity against recombinant S-adenosyl L-Methionine synthetase (alpha-isoform) in rat liverMore data for this Ligand-Target Pair

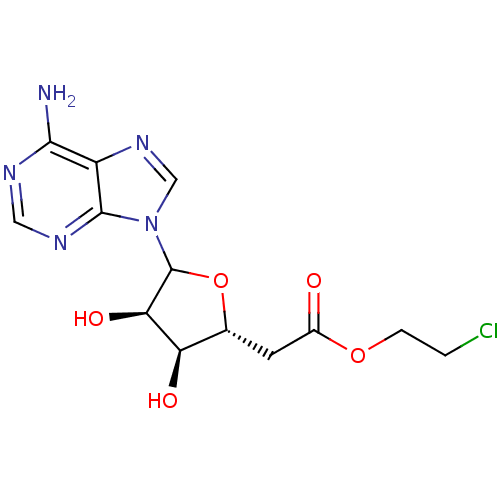
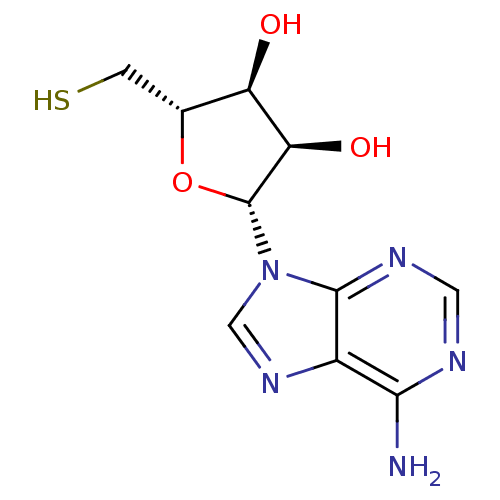
 3D Structure (crystal)
3D Structure (crystal)