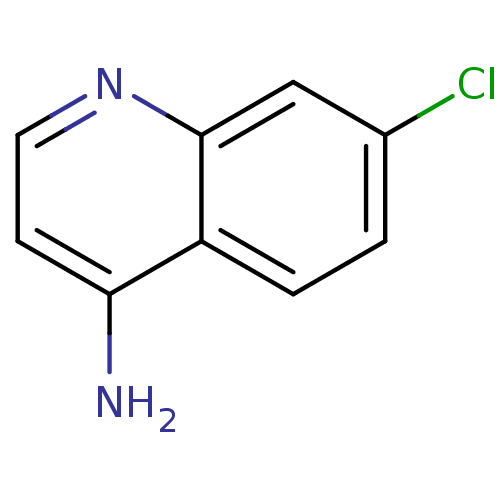
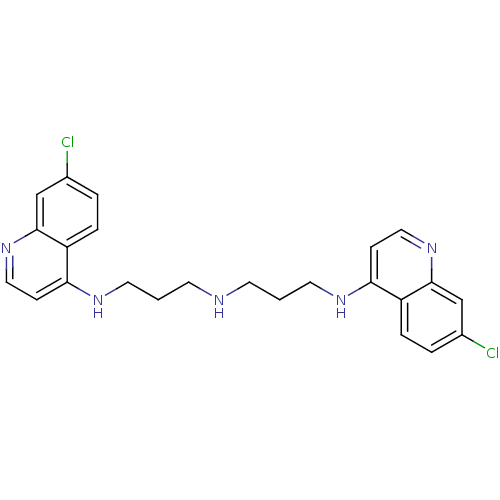
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 300nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 400nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 8.00E+3nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 1.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 3.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: 6.00E+4nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum (strain Hall / ATCC 3502 / N...)
National Cancer Institute-Frederick
Curated by ChEMBL
National Cancer Institute-Frederick
Curated by ChEMBL
Affinity DataKi: >3.00E+5nMAssay Description:Inhibition of BoNT/A light chain metalloprotease activityMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 3.20E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 7.00E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataIC50: 1.13E+4nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DataIC50: 1.70E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A(Clostridium botulinum)
National Cancer Institute At Frederick
Curated by ChEMBL
National Cancer Institute At Frederick
Curated by ChEMBL
Affinity DataIC50: >2.90E+4nMAssay Description:Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assayMore data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
TargetBotulinum neurotoxin type A2 [1-425](Clostridium botulinum)
National Cancer Institute At Frederick
National Cancer Institute At Frederick
Affinity DatapH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair

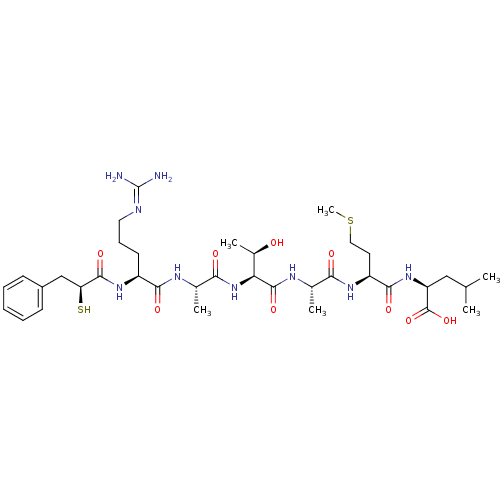


 3D Structure (crystal)
3D Structure (crystal)