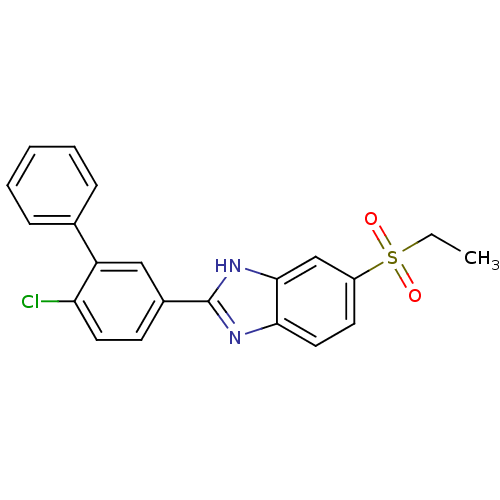
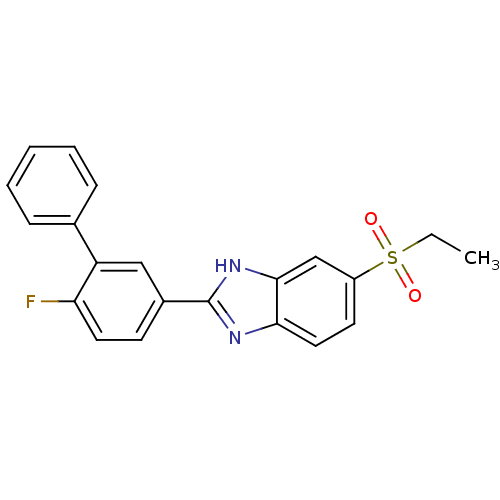
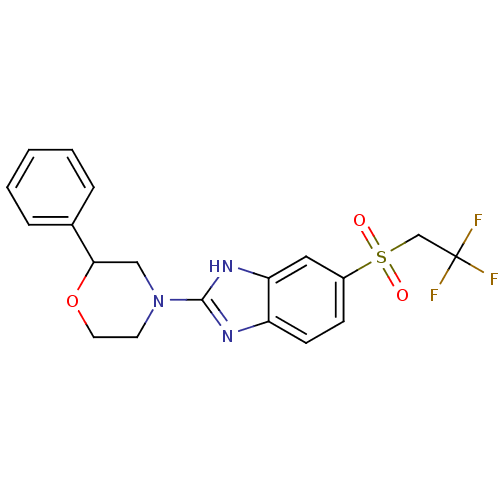
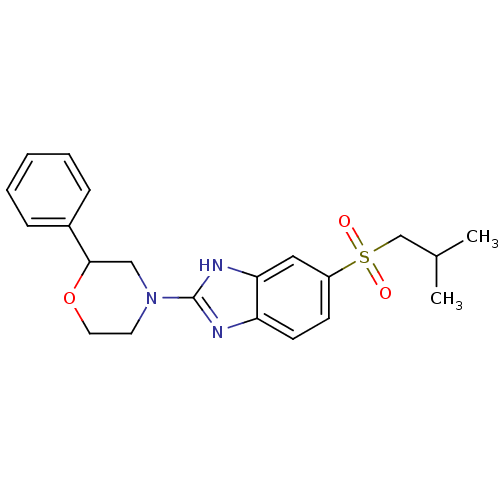
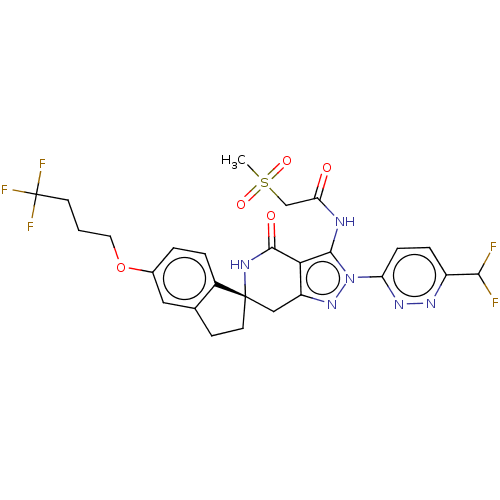
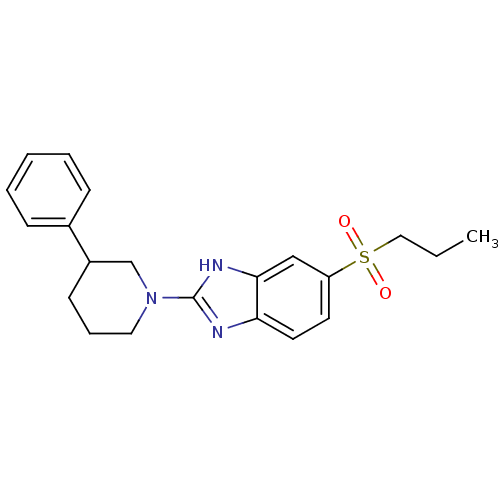
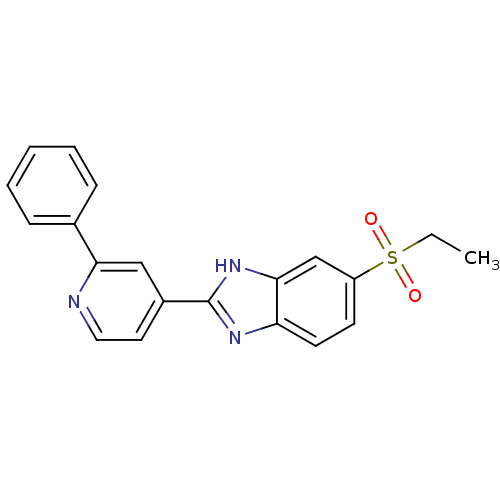
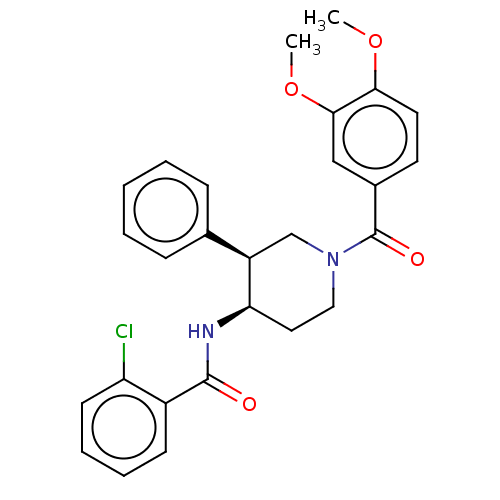
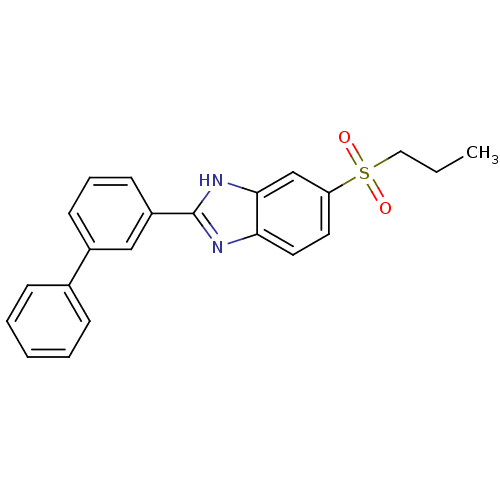
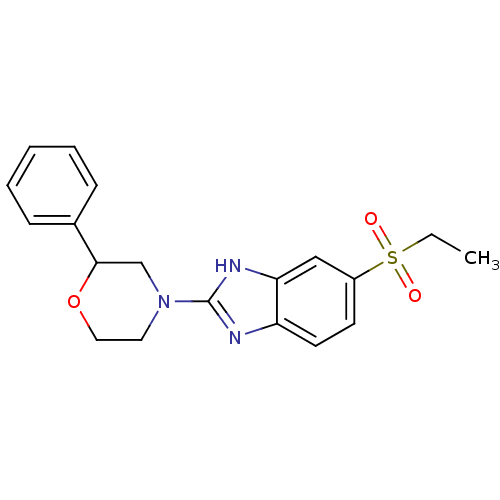
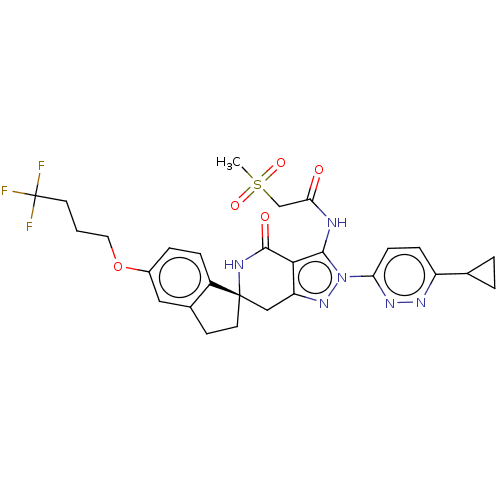
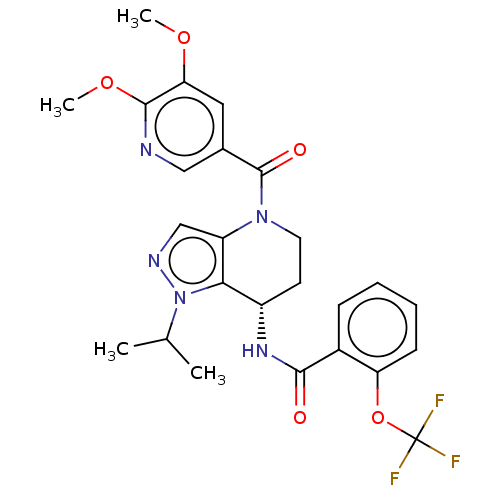
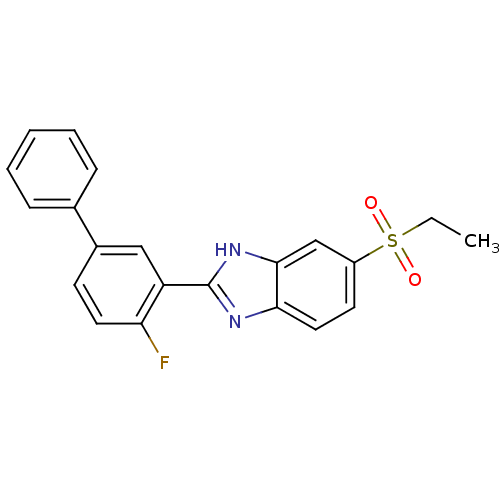
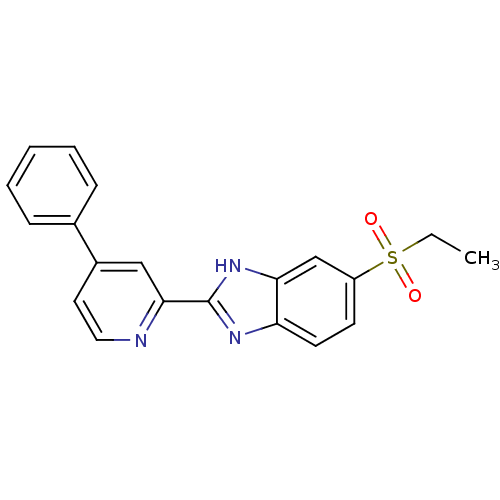
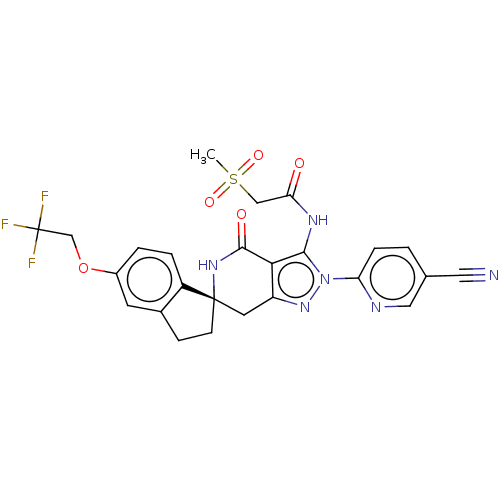
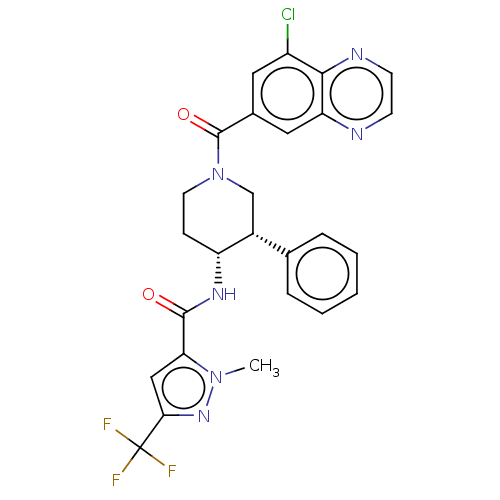
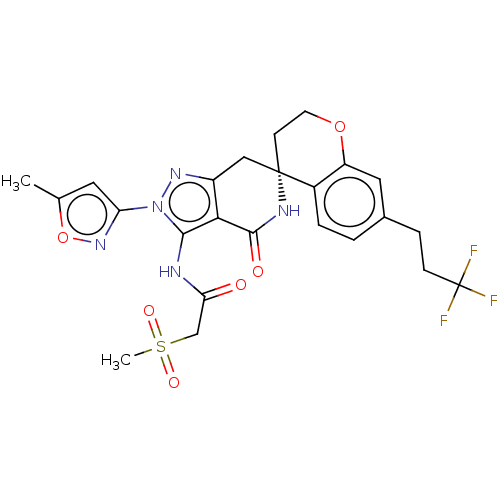
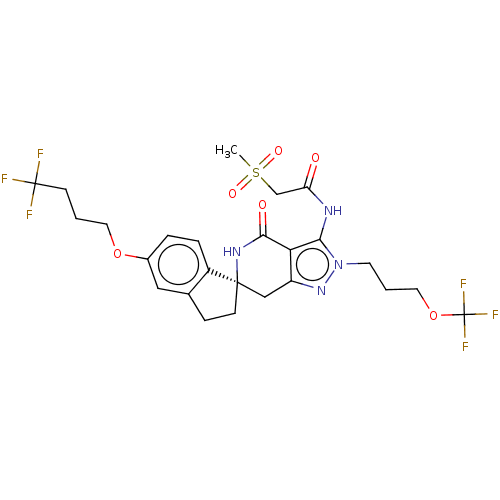
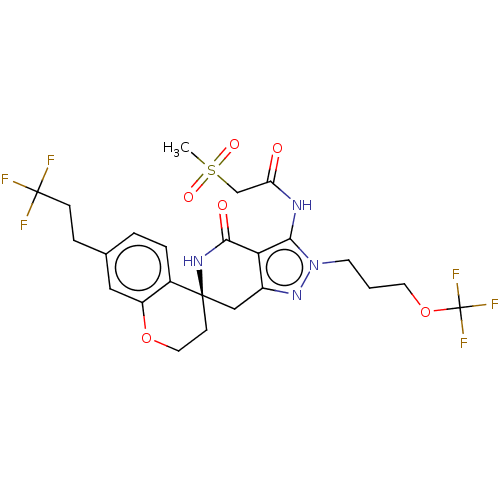
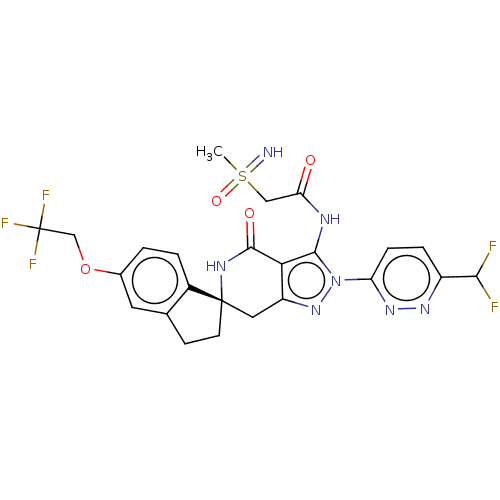
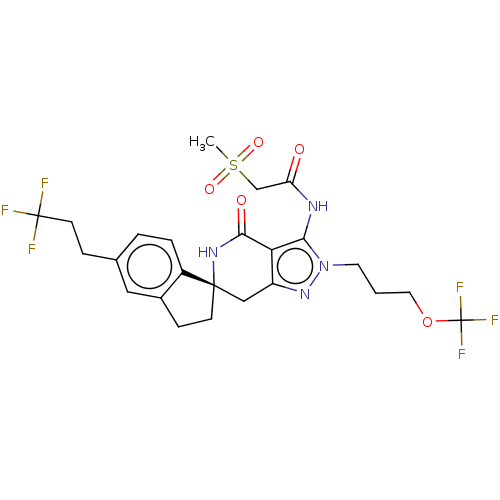
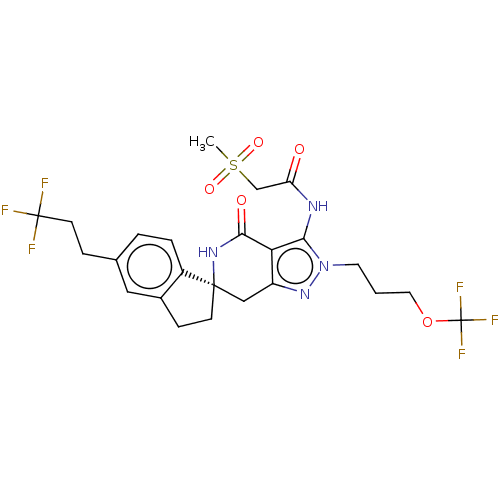
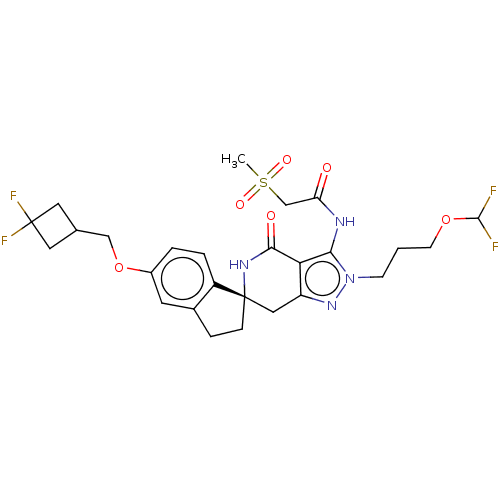
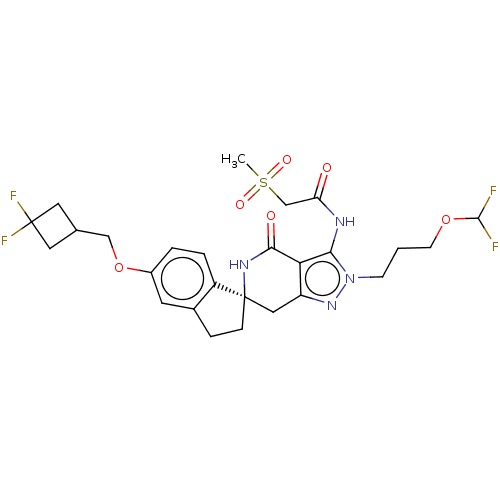
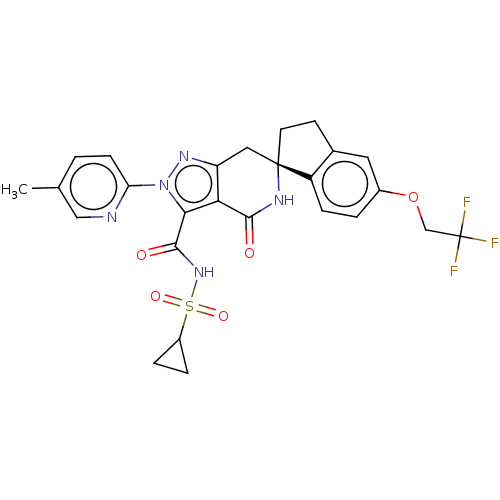
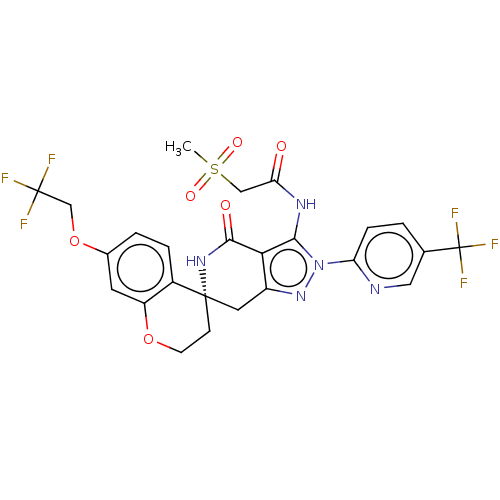
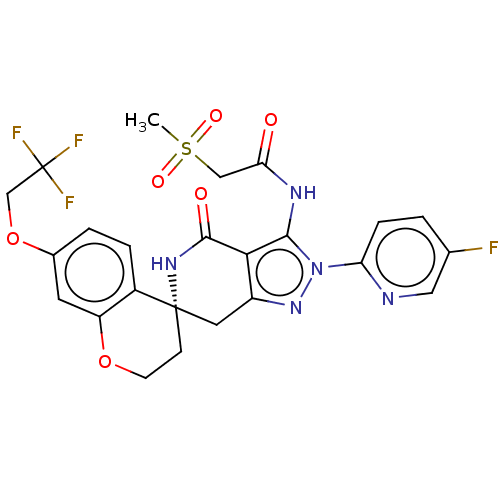
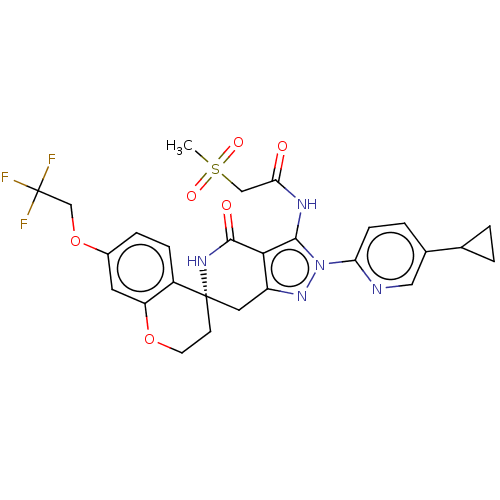
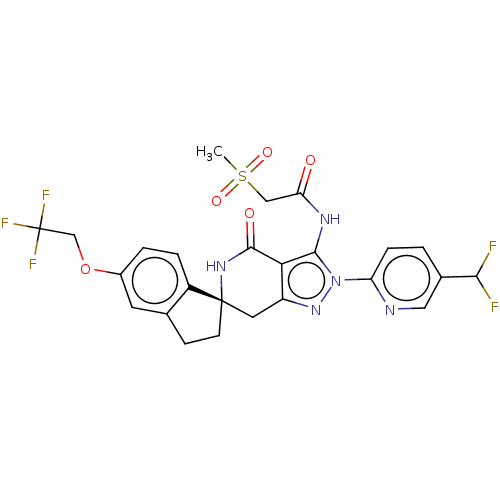
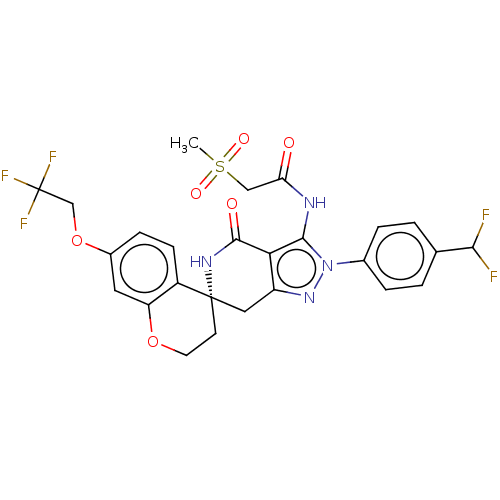
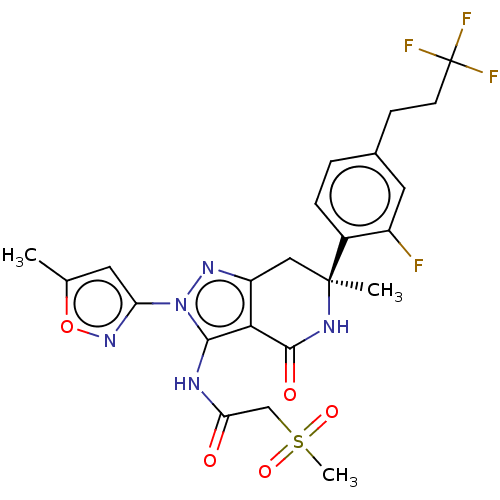
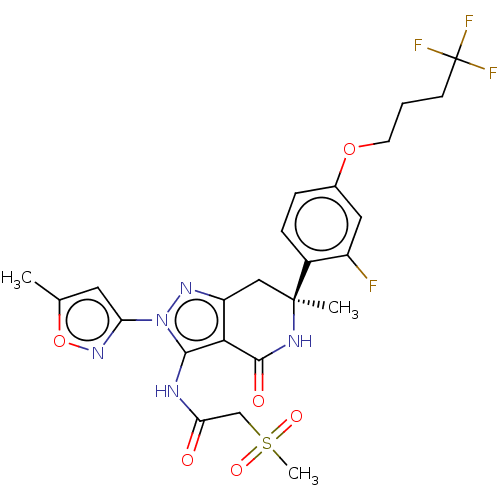
TargetHigh affinity nerve growth factor receptor(Homo sapiens (Human))
Takeda Pharmaceutical
Curated by ChEMBL
Takeda Pharmaceutical
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as...More data for this Ligand-Target Pair
Affinity DataIC50: 0.130nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.160nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.200nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.210nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.224nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.280nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.285nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.290nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.310nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.360nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.409nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.430nMAssay Description:Displacement of [125I]PYY from mouse NPY-Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.439nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.460nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.540nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.550nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.600nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.620nMAssay Description:A full-length human MGAT2 gene to which a Flag-tag had been added at the N-terminal was inserted into pFastBac (from Invitrogen). A recombinant bacul...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.706nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
Affinity DataIC50: 0.710nMAssay Description:Inhibition of human SPT2 transfected in Freestyle293 cells using L-serine and palmitoyl-CoA as substrate preincubated for 60 mins followed by substra...More data for this Ligand-Target Pair
Affinity DataIC50: 0.730nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 0.730nMAssay Description:A full-length human MGAT2 gene to which a Flag-tag had been added at the N-terminal was inserted into pFastBac (from Invitrogen). A recombinant bacul...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.850nMAssay Description:Displacement of [125I]PYY from mouse NPY Y5 receptorMore data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: <1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:Solutions of the compounds of the present invention in DMSO were each aliquoted into 0.2-μL portions in a 384-well polystyrene microplate produc...More data for this Ligand-Target Pair
TargetAlpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase(Homo sapiens (Human))
Shionogi
US Patent
Shionogi
US Patent
Affinity DataIC50: 1nMAssay Description:A full-length human MGAT2 gene to which a Flag-tag had been added at the N-terminal was inserted into pFastBac (from Invitrogen). A recombinant bacul...More data for this Ligand-Target Pair

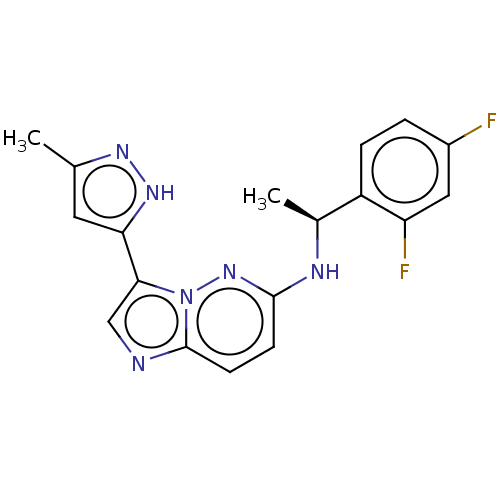
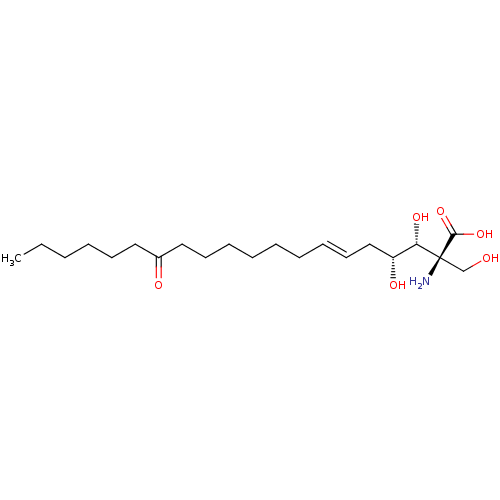
 3D Structure (crystal)
3D Structure (crystal)