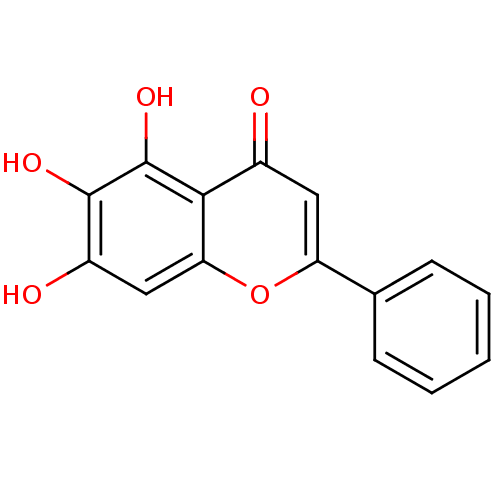
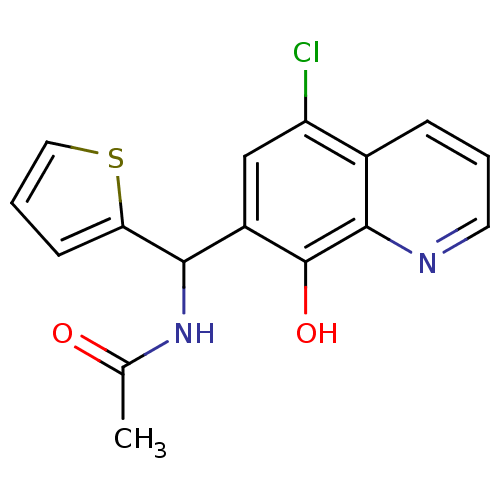
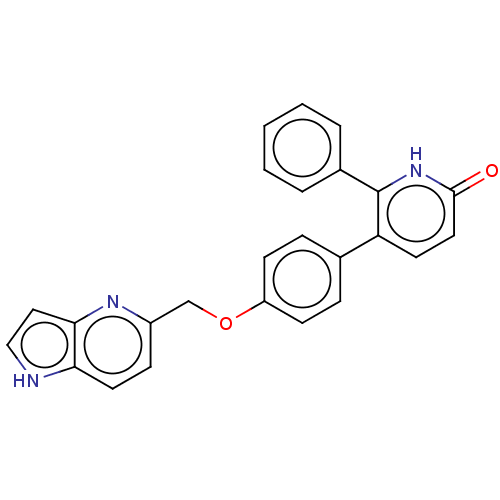
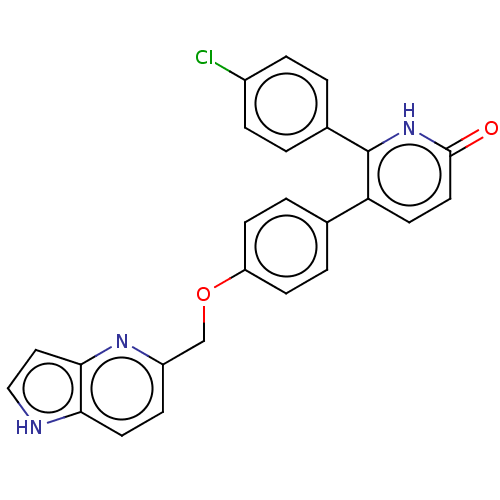
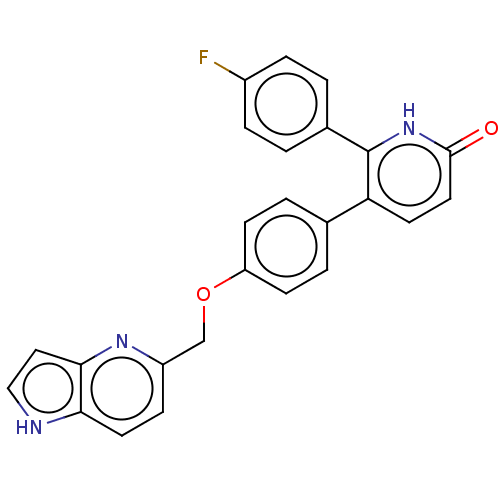
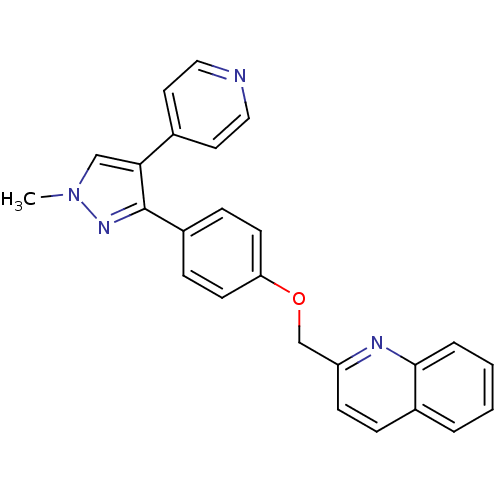
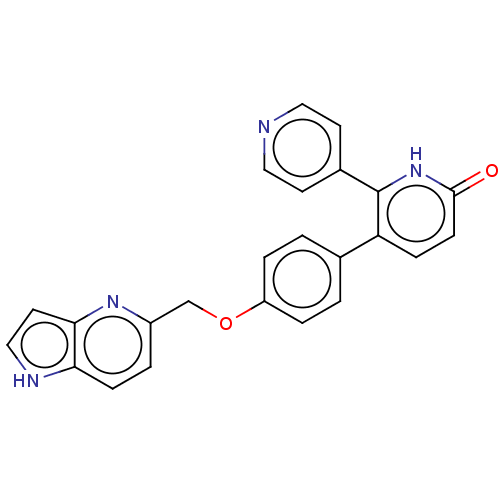
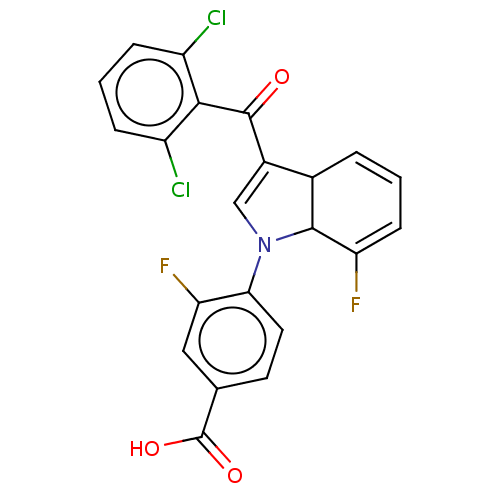
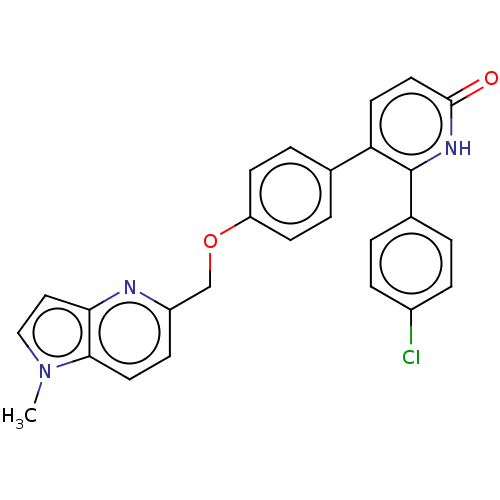
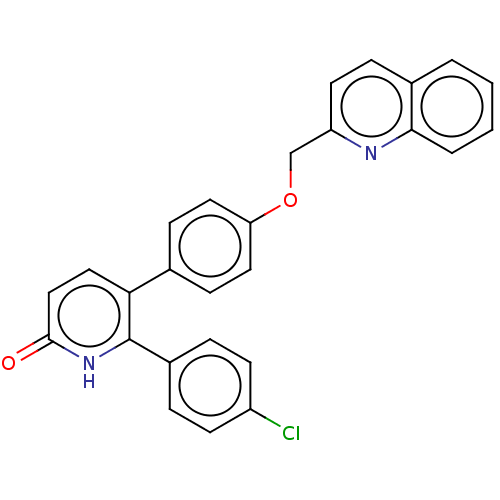
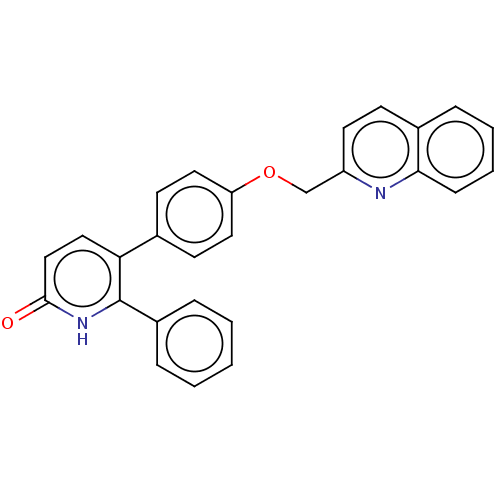
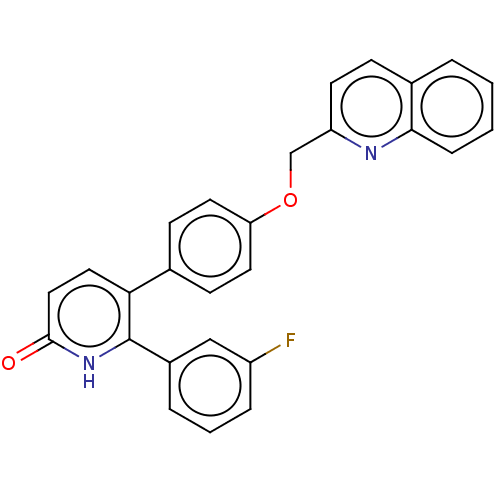
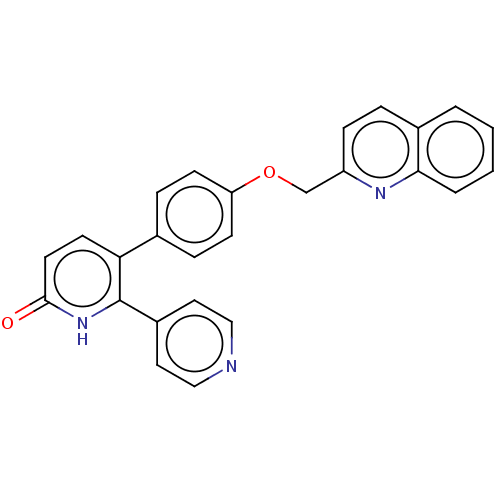
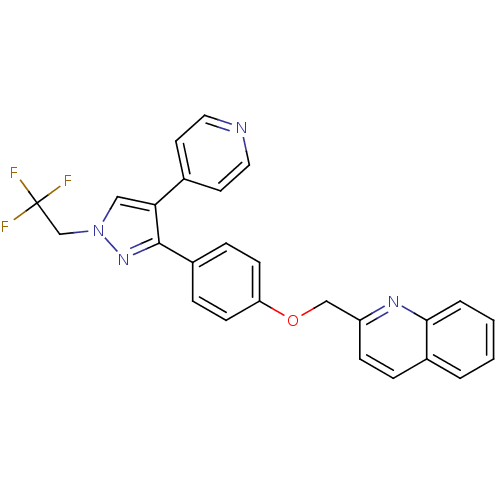
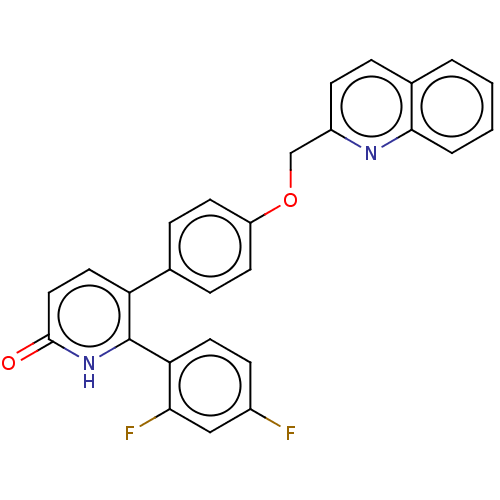
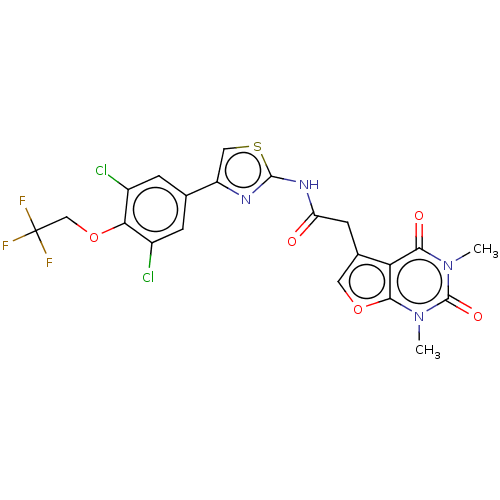
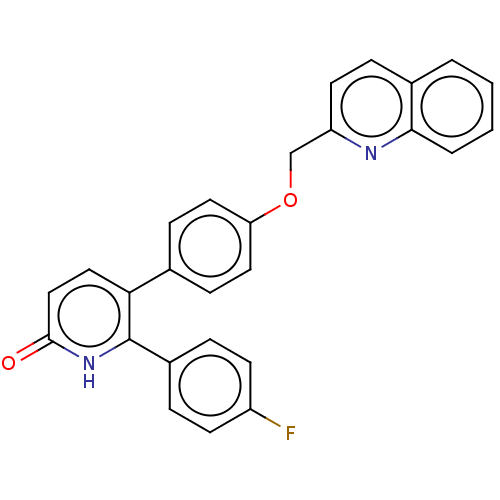
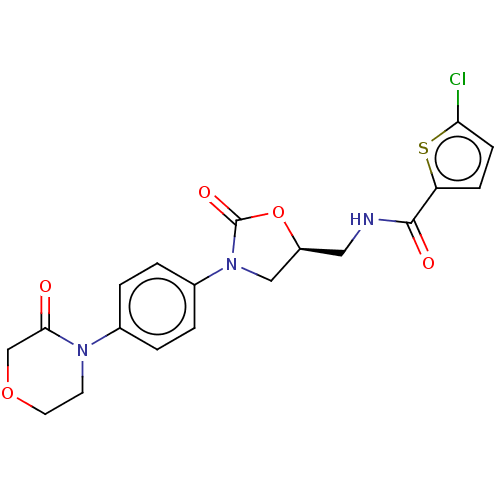
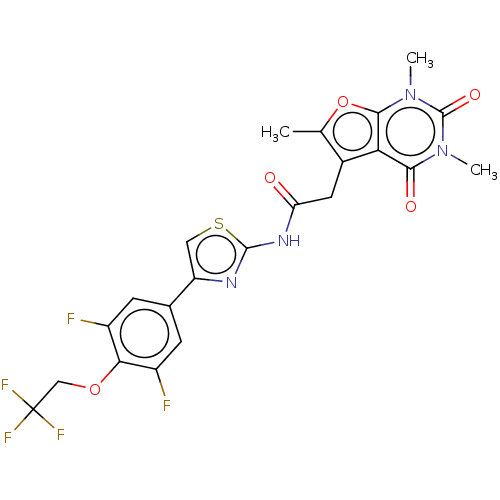
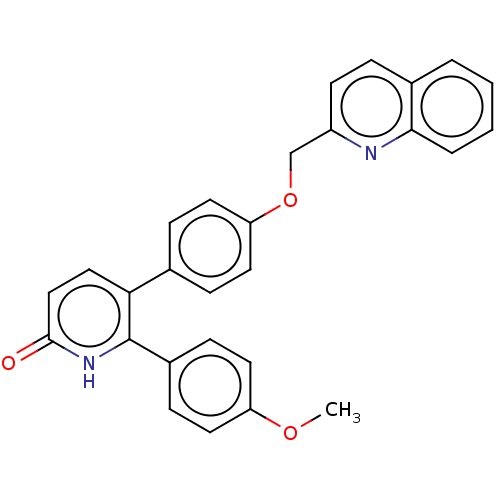
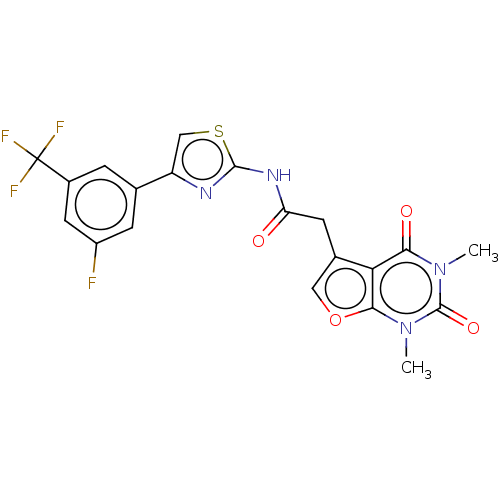
Affinity DataKi: 1.10nMAssay Description:Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of F10a assessed as S-2765 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataKi: 100nMAssay Description:Inhibition of recombinant N-terminal His6-tagged human reticulocyte 12/15-lipoxygenase using arachidonic acid as substrate assessed as equilibrium co...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 350nMAssay Description:Uncompetitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 530nMAssay Description:Uncompetitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataKi: 600nMAssay Description:Inhibition of human 15-lipoxygenaseMore data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 620nMAssay Description:Competitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-Mi...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 700nMAssay Description:Competitive inhibition of human platelet-type N-terminally His6-tagged 12-lipoxygenase assessed as 12-HPETE formation using arachidonic acid by by Mi...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 720nMAssay Description:Competitive inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate assessed as 12-HPETE formation by Henri-Mi...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX15(Homo sapiens (Human))
Harvard Medical School
Curated by ChEMBL
Harvard Medical School
Curated by ChEMBL
Affinity DataKi: 1.20E+3nMAssay Description:Inhibition of recombinant N-terminal His6-tagged human reticulocyte 12/15-lipoxygenase using arachidonic acid as substrate assessed as equilibrium co...More data for this Ligand-Target Pair
TargetPolyunsaturated fatty acid lipoxygenase ALOX12(Homo sapiens (Human))
National Institutes Of Health
Curated by ChEMBL
National Institutes Of Health
Curated by ChEMBL
Affinity DataKi: 1.60E+3nMAssay Description:Inhibition of human platelet-type 12-lipoxygenaseMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of tPA assessed as S-2288 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of thrombin assessed as S-2238 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of plasmin assessed as S-2302 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+4nMAssay Description:Inhibition of activated protein C assessed as S-2366 substrate hydrolysis by microplate reader analysisMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.210nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.300nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.330nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.370nMAssay Description:Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.540nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.615nMAssay Description:The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.730nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Rattus norvegicus (rat))
Glenmark Pharmaceuticals
Curated by ChEMBL
Glenmark Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 0.800nMAssay Description:Inhibition of rat recombinant GST-tagged PDE10A using [3H]-cAMP as substrate by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of human recombinant PDE10A using [3H]-cAMP/[3H]-cGMP as substrate after 30 mins by radiometric assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.10nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Mus musculus)
Glenmark Pharmaceuticals
Curated by ChEMBL
Glenmark Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of mouse recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.30nMAssay Description:Inhibition of human recombinant GST-tagged PDE10A using [3H]-cAMP as substrate after 30 mins by scintillation proximity assayMore data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of N-terminal GST-tagged human recombinant PDE10A assessed as substrate hydrolysis using [3H]cAMP as substrate after 30 mins by two-step r...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.40nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Glenmark Pharmaceuticals
US Patent
Glenmark Pharmaceuticals
US Patent
Affinity DataIC50: 1.45nMT: 2°CAssay Description:The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.60nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human F10a using S-2765 as substrate after 45 minsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Glenmark Pharmaceuticals
US Patent
Glenmark Pharmaceuticals
US Patent
Affinity DataIC50: 1.68nMT: 2°CAssay Description:The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.70nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of recombinant human mPGES-1 expressed in CHO cells assessed as reduction in PGE2 formation using PGH2 a substrate preincubated for 10 min...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
TargetcAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A(Homo sapiens (Human))
Glenmark Research Centre
Curated by ChEMBL
Glenmark Research Centre
Curated by ChEMBL
Affinity DataIC50: 1.90nMAssay Description:Inhibition of recombinant N-terminal GST-tagged human PDE10A using [3H]cAMP/cAMP as substrate assessed as hydrolysis of [3H]cAMP to [3H]AMP after 30 ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human recombinant mPGES-1 expressed in CHO cells assessed as reduction in conversion of PGH2 to PGE2 incubated for 10 mins followed by ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.98nMAssay Description:The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding...More data for this Ligand-Target Pair
Affinity DataIC50: 2.10nMAssay Description:Inhibition of human F10a using S-2765 as substrate after 45 minsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily A member 1(Homo sapiens (Human))
Glenmark Pharmaceuticals
US Patent
Glenmark Pharmaceuticals
US Patent
Affinity DataIC50: 2.15nMT: 2°CAssay Description:The inhibition of TRPA1 receptor activation was measured as inhibition of allylisothiocyanate (AITC) induced cellular uptake of radioactive calcium. ...More data for this Ligand-Target Pair
Affinity DataIC50: 2.18nMAssay Description:The assay is based on the principle that binding of the agonist to the RORγ causes a conformational change around helix 12 in the ligand binding...More data for this Ligand-Target Pair


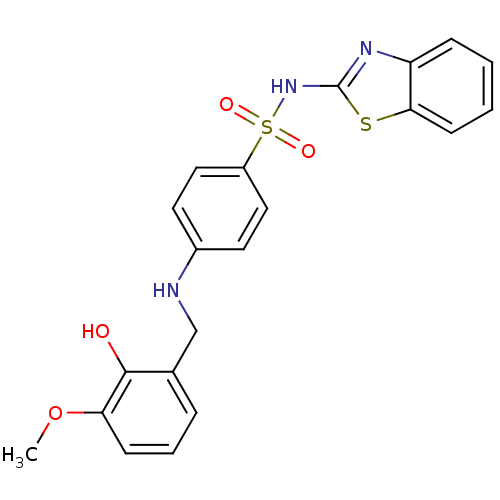
 3D Structure (crystal)
3D Structure (crystal)