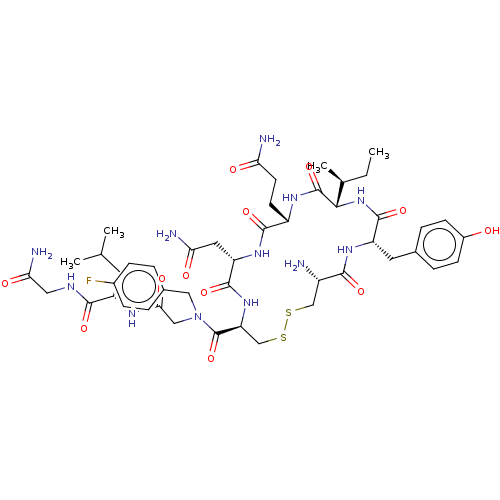
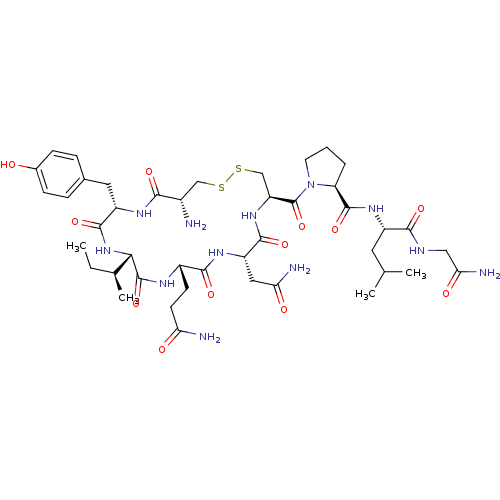
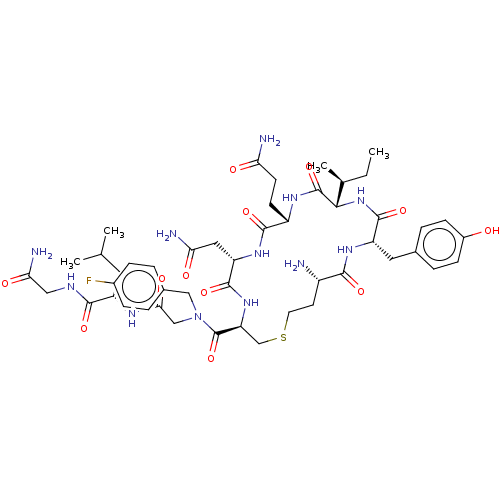
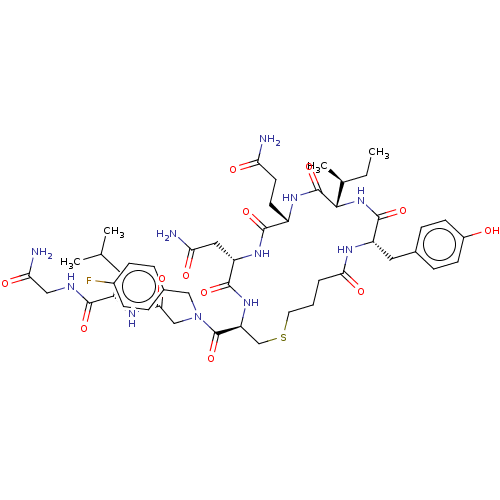
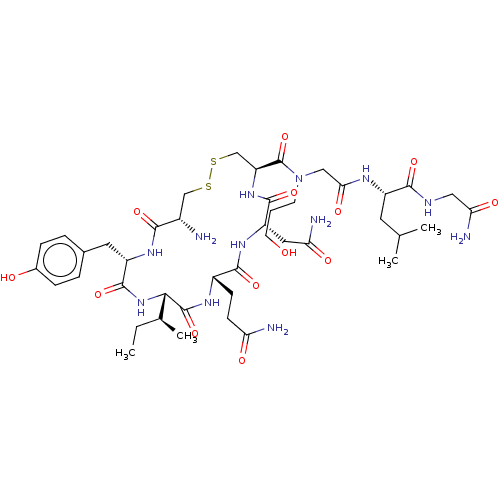
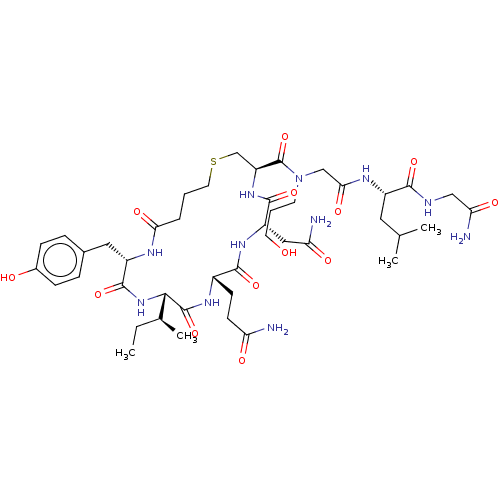
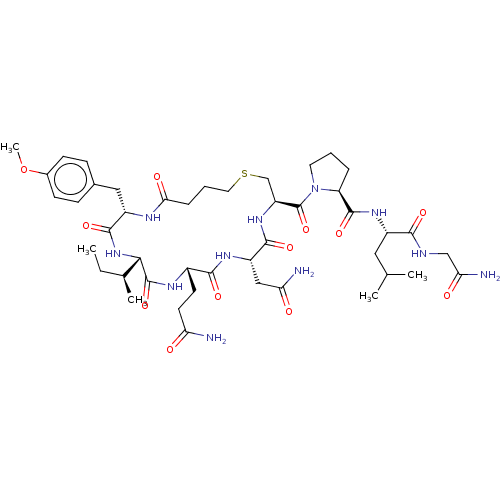
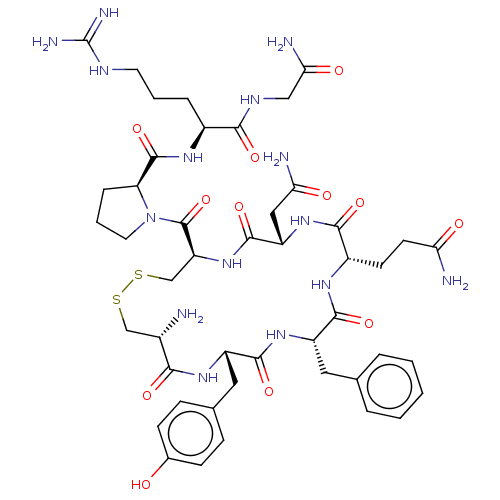
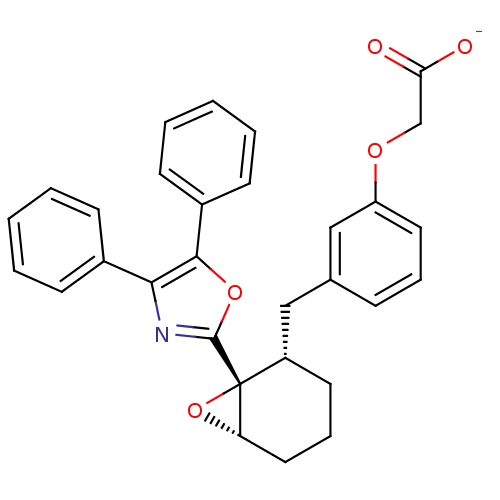
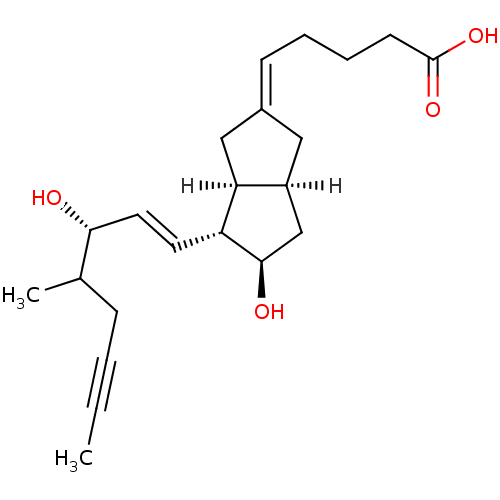
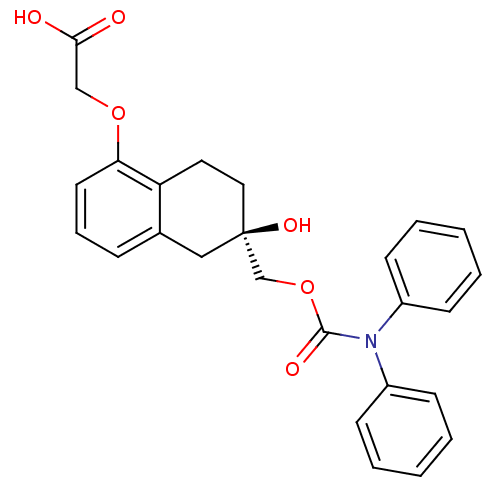
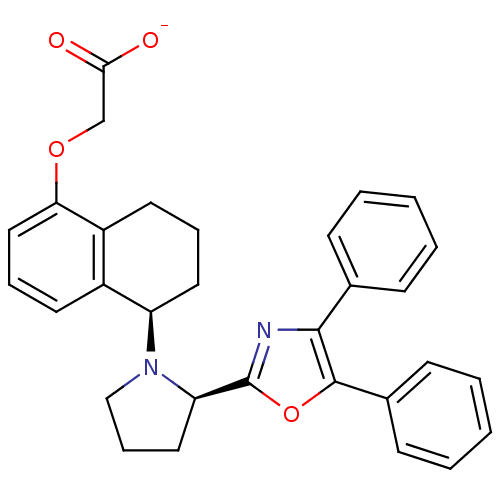
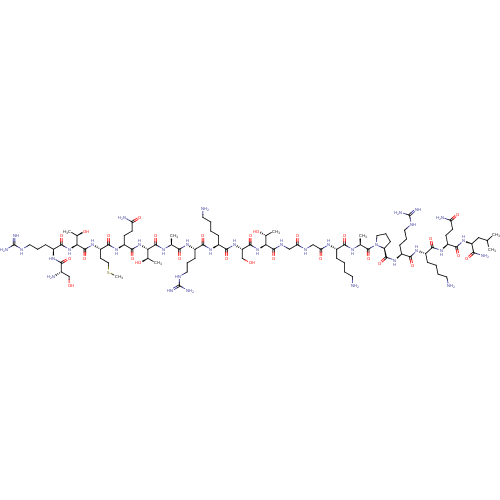
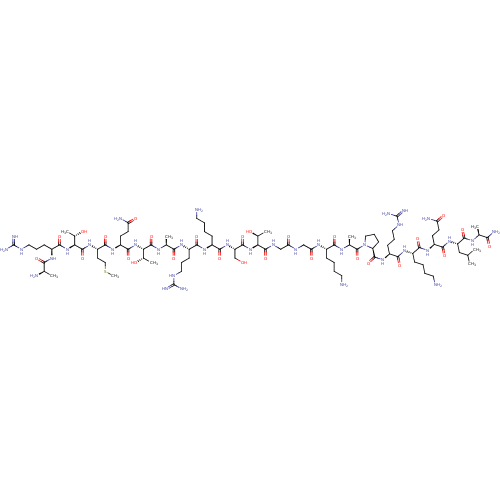
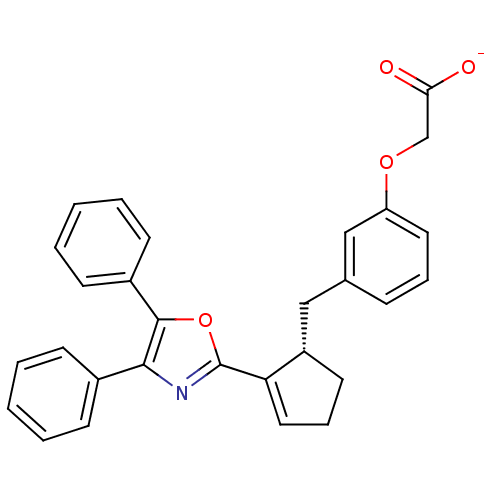
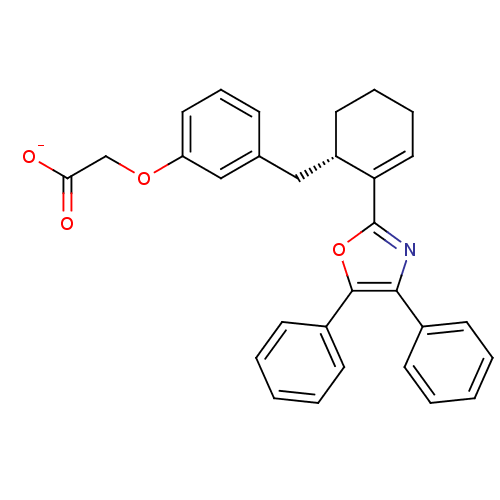
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.510nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.580nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 0.900nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.10nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 1.80nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
TargetOxytocin receptor(Homo sapiens (Human))
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Tohoku University And Department Of Pharmaceutical Sciences
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]OT from recombinant human OTR expressed in HEK293 cell membranes measured after 1 hrMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.5nMAssay Description:Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.90nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptorMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 30nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Inhibition of human LSD1 assessed as reduction in H2O2 production using pLys4Met H3 peptide as substrate by peroxidase coupled UV-visible spectrophot...More data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Ability to inhibit binding of [3H]iloprost to cloned human prostaglandin I2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of [3H]-Iloprost binding to human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 60nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 98nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Displacement of [3H]iloprost from human Prostanoid IP receptorMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 290nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 380nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
Affinity DataKi: 930nMAssay Description:Inhibition of [3H]-SQ-29,548 binding to human Prostanoid TP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-SQ-29,548 binding to human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to Prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGD-2 binding to human Prostanoid DP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGF-2 binding to human Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to Prostanoid EP1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGD-2 from human Prostanoid DP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]SQ-29,548 from human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGD-2 from human Prostanoid DP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGF-2 from human Prostanoid FP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP1 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP1 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]-PGE-2 from human Prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]PGF-2 from human Prostanoid FP receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Displacement of [3H]SQ-29,548 from human Prostanoid TP receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.02E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP4 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.02E+3nMAssay Description:Displacement of [3H]PGE-2 from human Prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetLysine-specific histone demethylase 1A(Homo sapiens (Human))
Nagoya City University
Curated by ChEMBL
Nagoya City University
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of human LSD1 (172 to 833 residues) assessed as reduction in H2O2 production using H3K4me2 (1 to 20 residues) peptide as substrate preincu...More data for this Ligand-Target Pair
TargetProstaglandin E2 receptor EP2 subtype(Homo sapiens (Human))
Fujisawa Pharmaceutical
Curated by ChEMBL
Fujisawa Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 1.40E+3nMAssay Description:Inhibition of [3H]-PGE-2 binding to Prostanoid EP2 receptorMore data for this Ligand-Target Pair