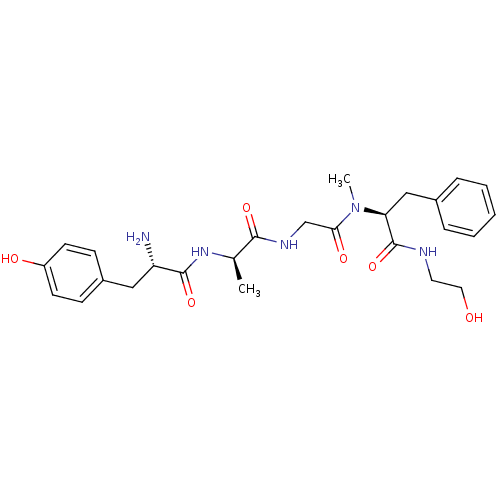
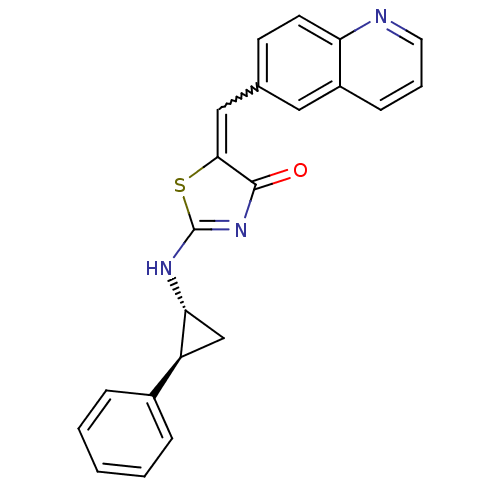
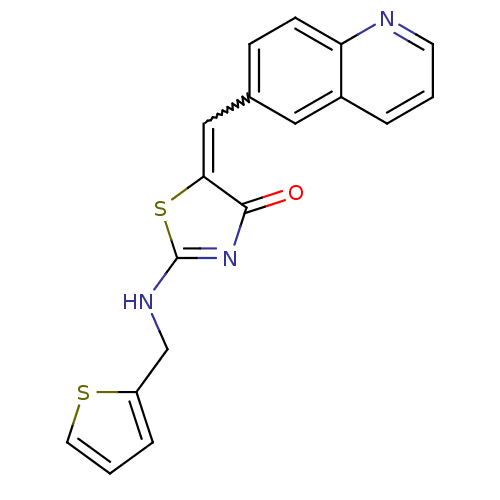
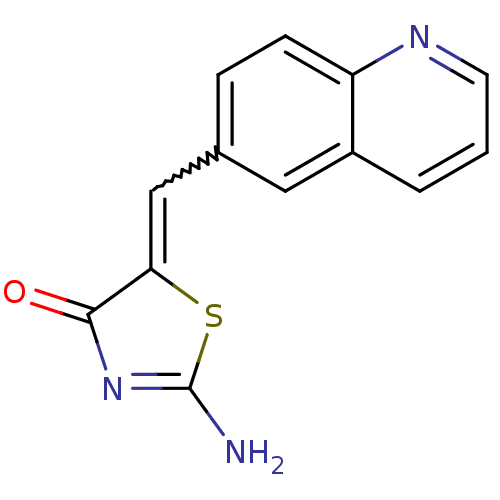
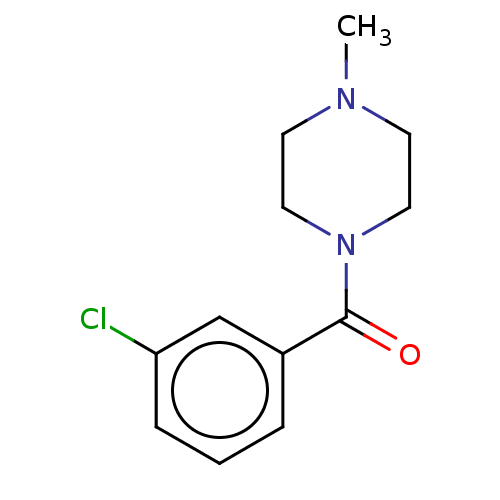
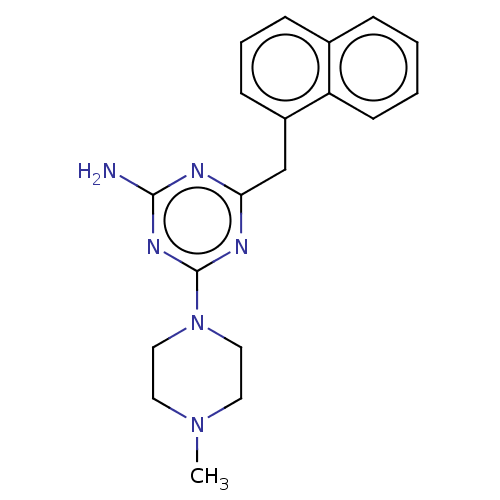
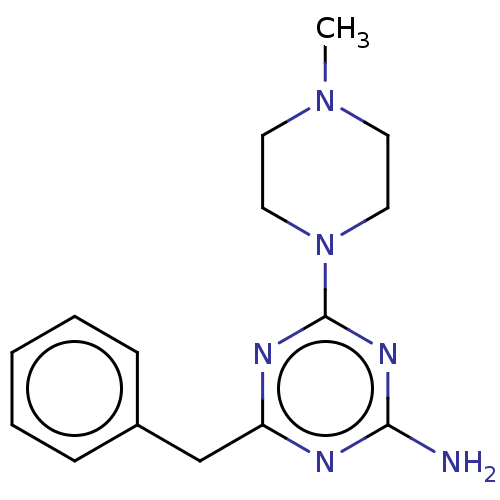
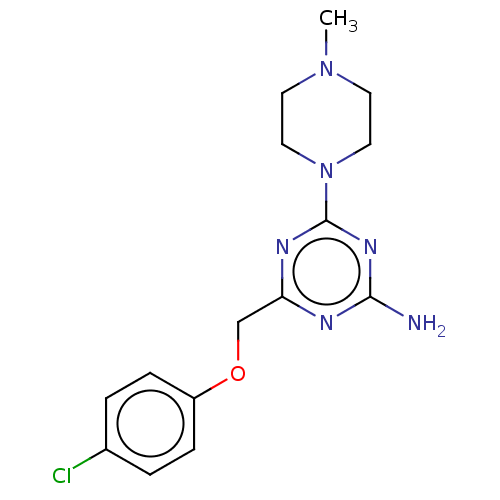
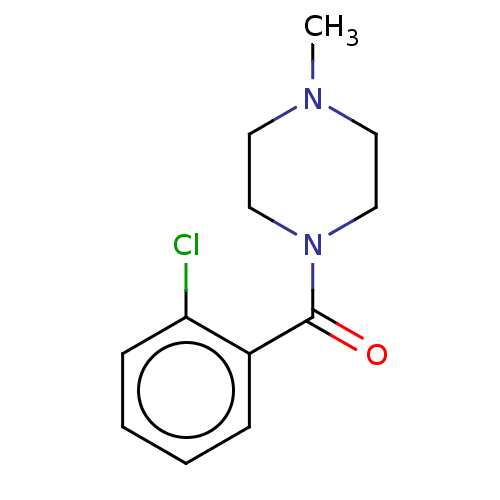
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMu-type opioid receptor(Homo sapiens (Human))
Molecular Research Institute
Curated by PDSP Ki Database
Molecular Research Institute
Curated by PDSP Ki Database
TargetMitogen-activated protein kinase 1(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of GSKp1More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of CDK4More data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase alpha-3(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKAP1More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of EPHB3More data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase alpha-3(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKAP1More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 1(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of CDK2More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of EPHB3More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of CDK4More data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of EPHB3More data for this Ligand-Target Pair
TargetRAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKCaMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKCaMore data for this Ligand-Target Pair
TargetRAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of GSKp1More data for this Ligand-Target Pair
TargetRAC-alpha/RAC-beta/RAC-gamma serine/threonine-protein kinase(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKCaMore data for this Ligand-Target Pair
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of CDK4More data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase alpha-3(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: >2.00E+3nMAssay Description:Inhibition of PKAP1More data for this Ligand-Target Pair
TargetMitogen-activated protein kinase 1(Homo sapiens (Human))
Roche Research Center
Curated by ChEMBL
Roche Research Center
Curated by ChEMBL
Affinity DataKi: 3.19E+3nM ΔG°: -29.2kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 3.52E+3nM ΔG°: -28.9kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 3.88E+3nM ΔG°: -28.7kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 5.60E+3nM ΔG°: -27.9kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 7.50E+3nM ΔG°: -27.2kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 7.55E+3nM ΔG°: -27.2kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 8.26E+3nM ΔG°: -27.0kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 1.06E+4nM ΔG°: -26.4kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: 1.46E+4nM ΔG°: -25.7kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-21.2kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nM ΔG°: >-21.2kJ/molepH: 7.4 T: 2°CAssay Description:Prior to the experiments, cell membranes were sedimented by a 10 min centrifugation at 4 °C
and 16 0009x g and resuspended in binding buffer (12...More data for this Ligand-Target Pair

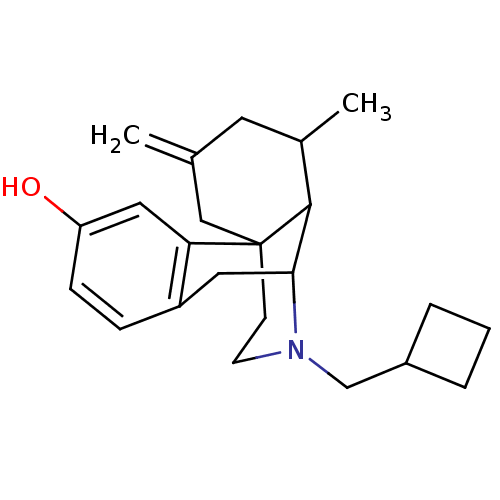
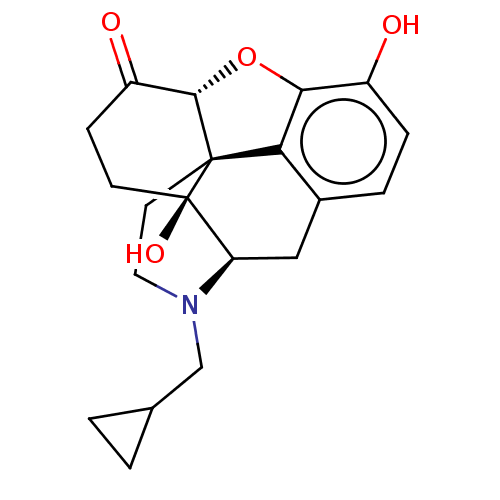
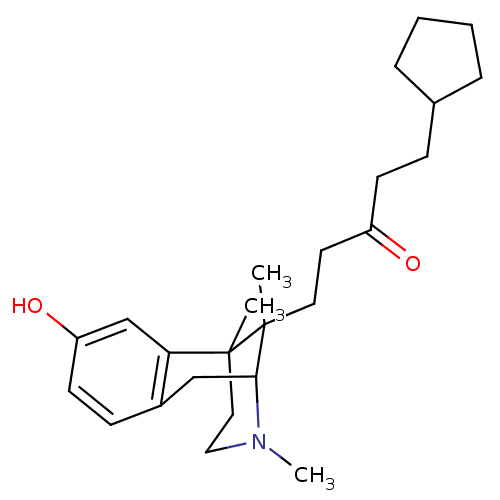
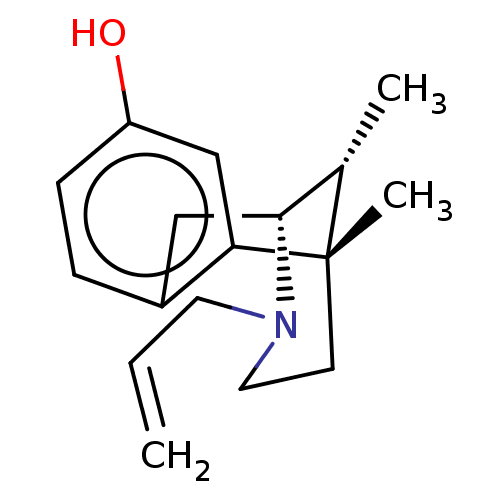
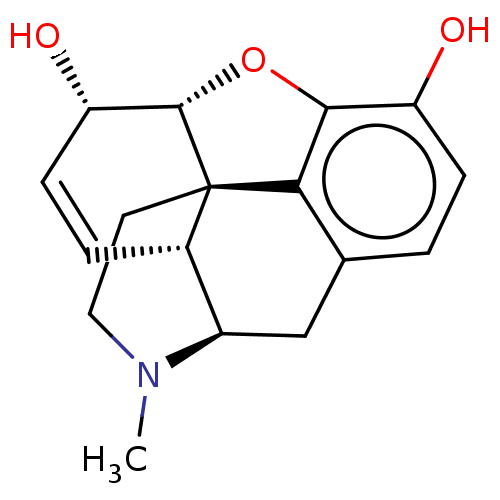
 3D Structure (crystal)
3D Structure (crystal)