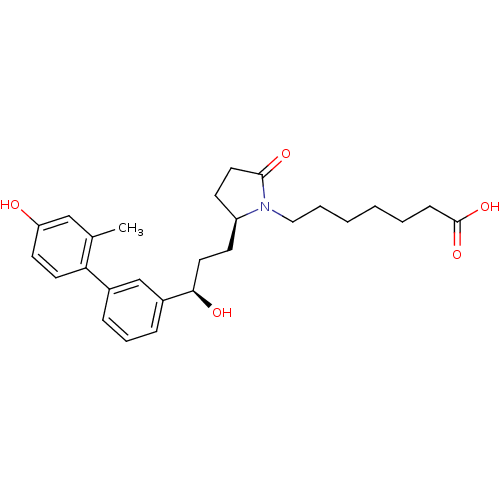
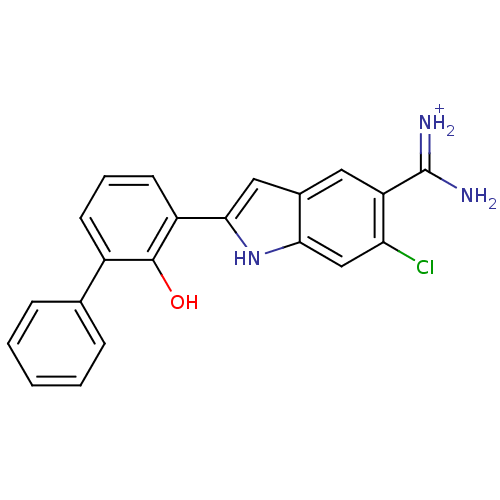
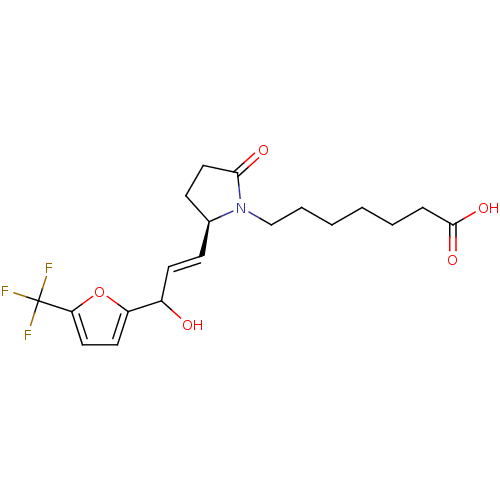
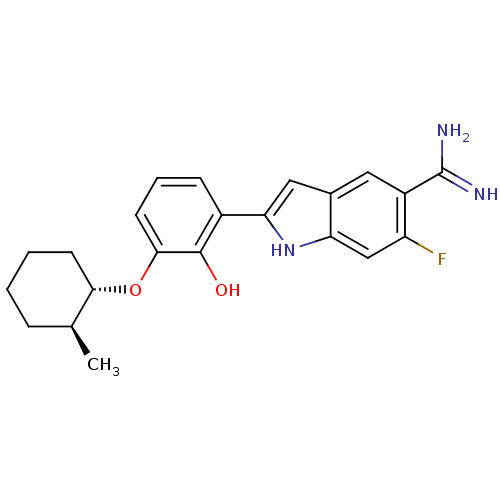
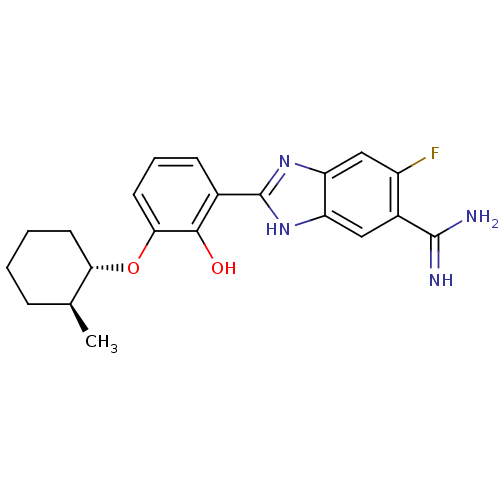
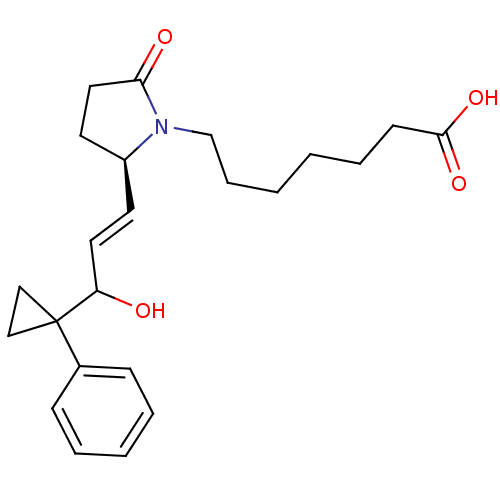
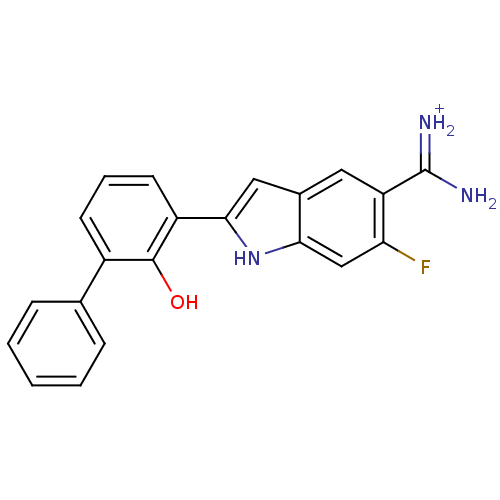
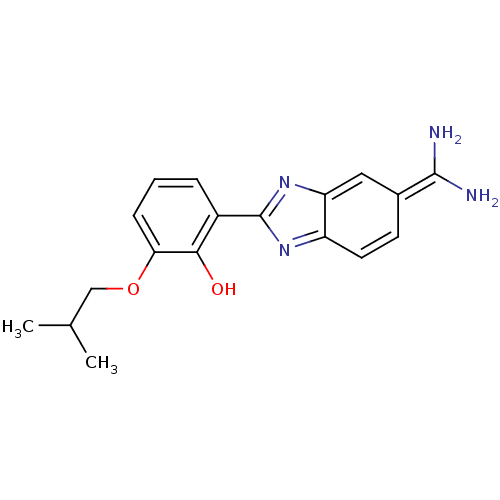
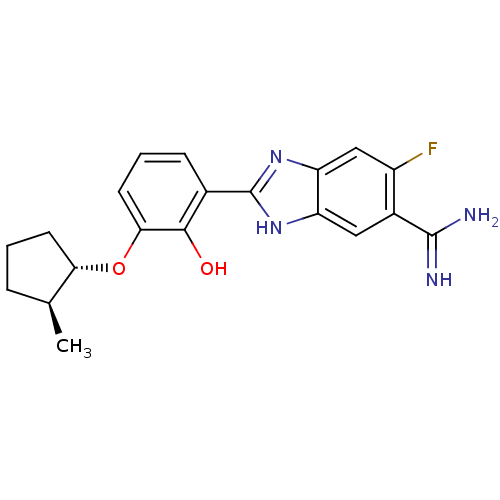
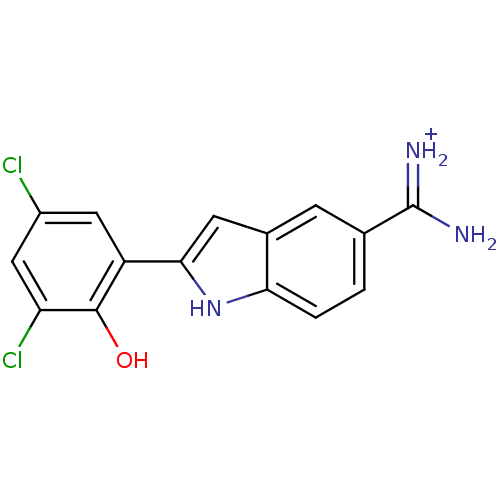
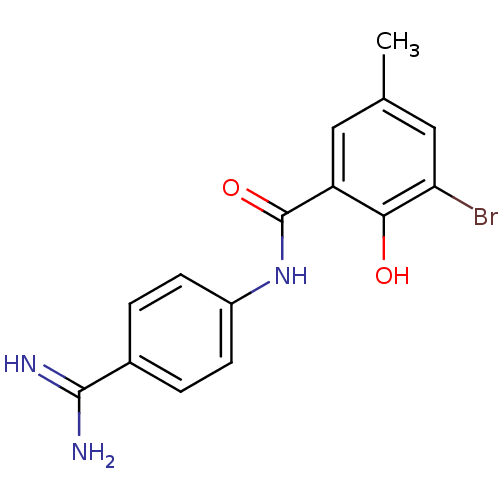
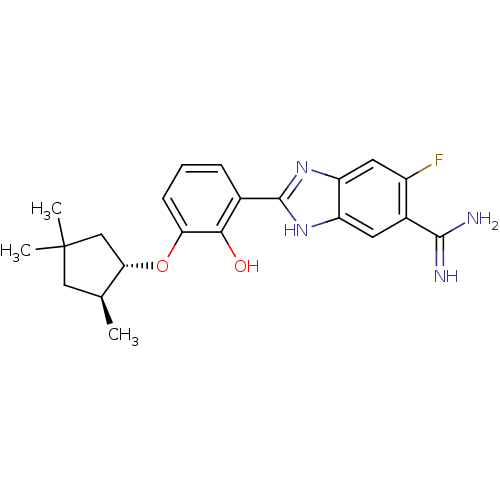
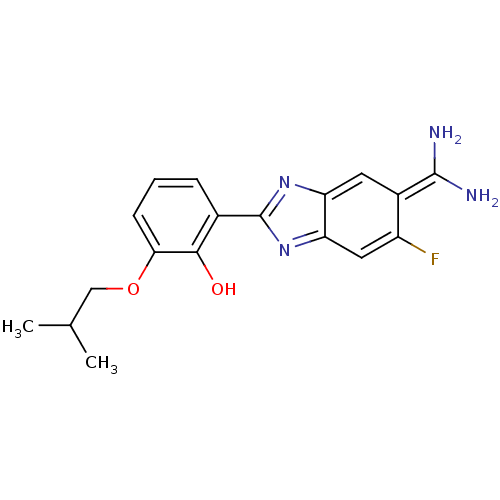
Affinity DataKi: 0.800nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Agonist activity against recombinant prostanoid EP4 receptor stably transfected in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.10nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.20nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Binding affinity was determined against prostanoid EP1 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.5nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 4.90nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.10nMAssay Description:Agonist activity against recombinant prostanoid EP4 receptor stably transfected in CHO cellsMore data for this Ligand-Target Pair
Affinity DataKi: 6.20nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 6.40nMAssay Description:Binding affinity was determined against prostanoid EP2 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.40nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 8nM ΔG°: -45.7kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Binding affinity was determined against prostanoid EP3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 8.20nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 9nM ΔG°: -45.5kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Burroughs Wellcome
Curated by ChEMBL
Burroughs Wellcome
Curated by ChEMBL
Affinity DataKi: 10nMAssay Description:In vitro binding affinity of the compound towards Monoamine Oxidase A in ratMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:In vitro binding affinity of the compound towards Monoamine Oxidase A in humanMore data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 11nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 12nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 20nM ΔG°: -43.5kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 25nM ΔG°: -43.0kJ/molepH: 8.2 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 32nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Inhibition of tissue-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 35nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 70nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 77nM ΔG°: -40.2kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Inhibition of Coagulation factor XMore data for this Ligand-Target Pair
Affinity DataKi: 78nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 80nMAssay Description:Inhibition of urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 85nMAssay Description:Inhibition of urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Inhibition of urokinase-type plasminogen activatorMore data for this Ligand-Target Pair
Affinity DataKi: 110nM ΔG°: -39.3kJ/molepH: 7.4 T: 2°CAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 110nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 130nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Binding affinity was determined against prostanoid EP4 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 140nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis...More data for this Ligand-Target Pair
Affinity DataKi: 150nMAssay Description:Inhibition of urokinase-type plasminogen activator (microPa)More data for this Ligand-Target Pair

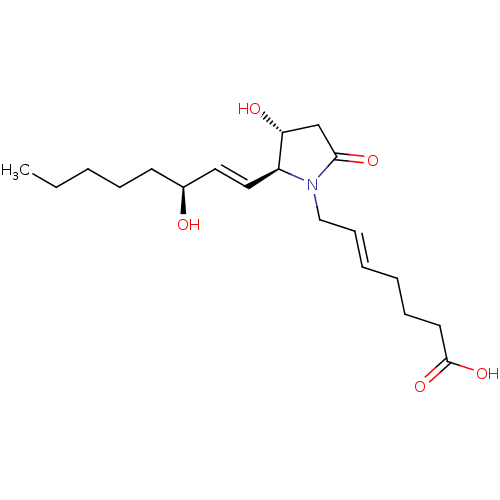



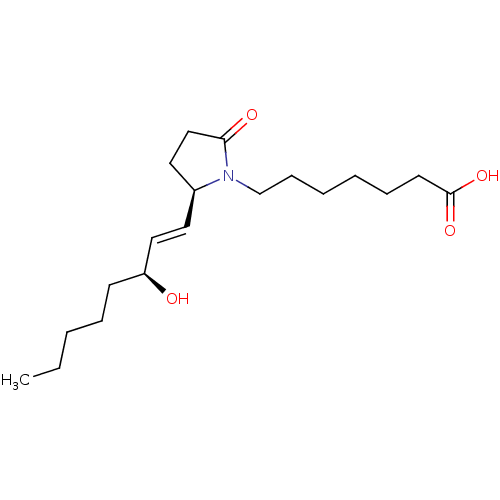
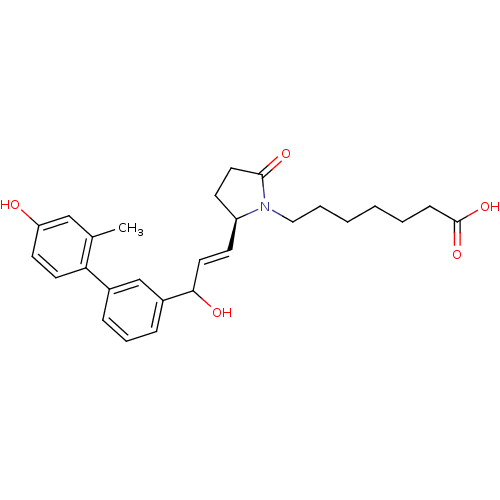
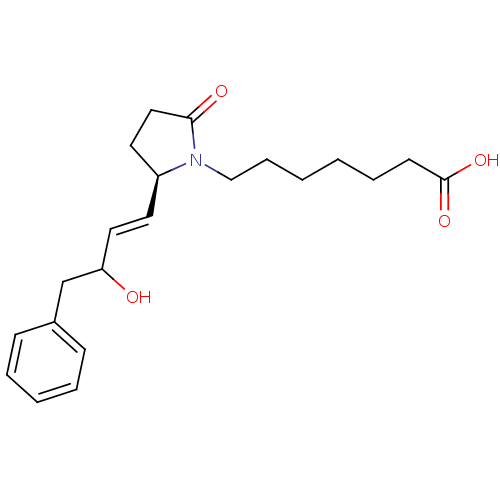

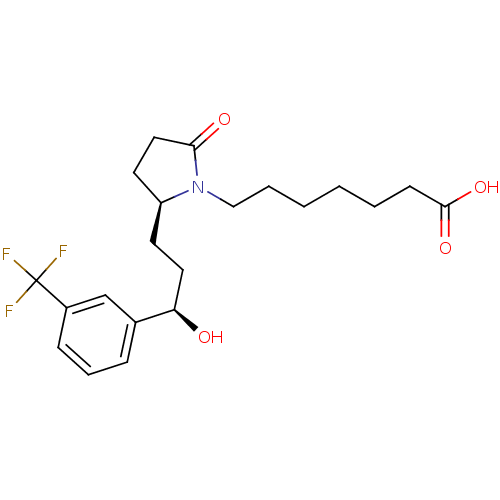

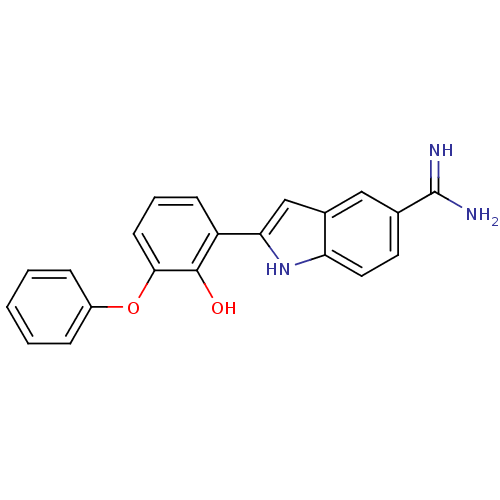
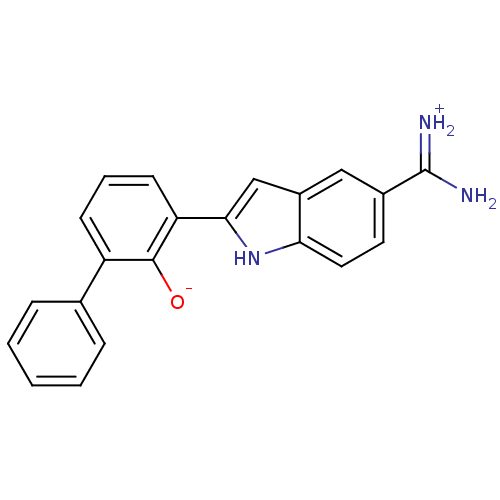
 3D Structure (crystal)
3D Structure (crystal)