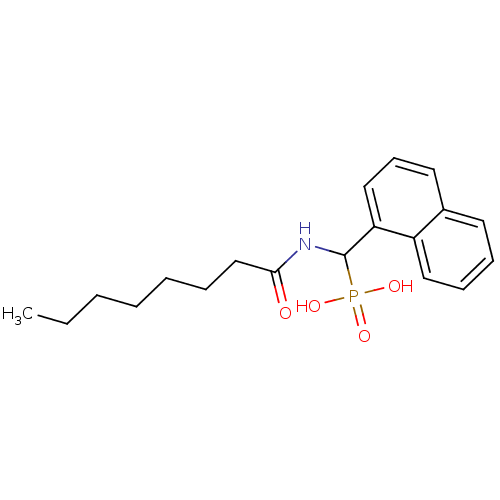
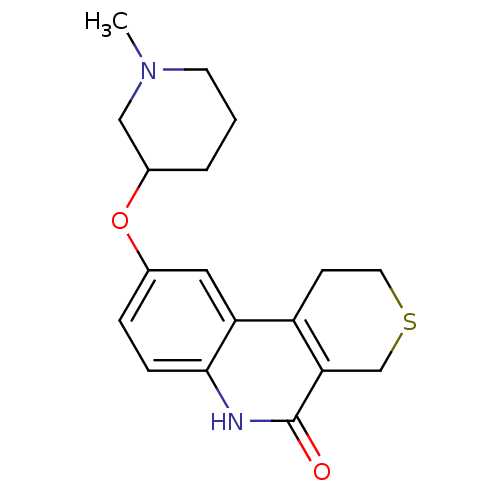
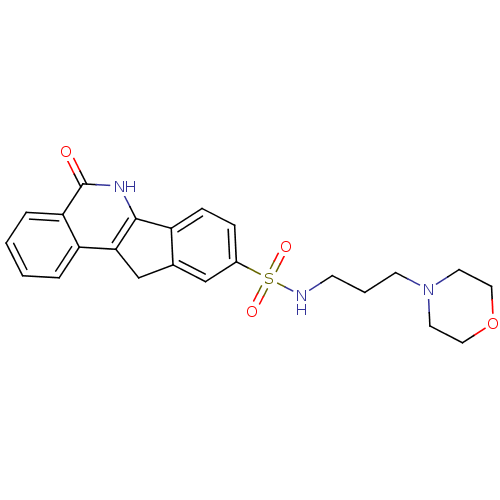
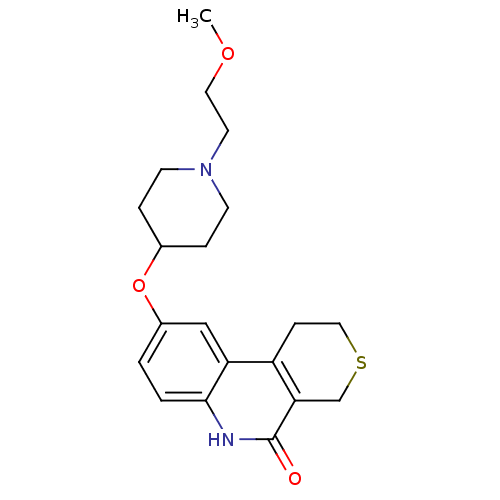
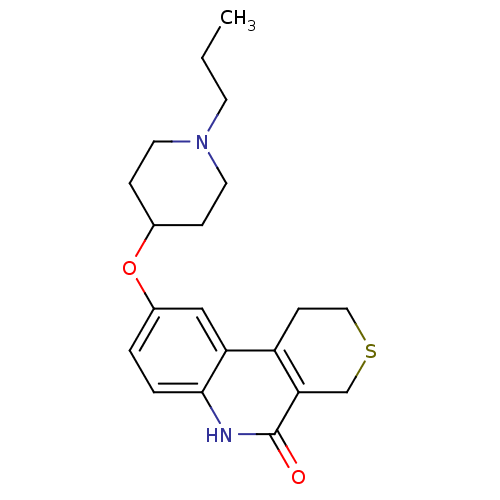
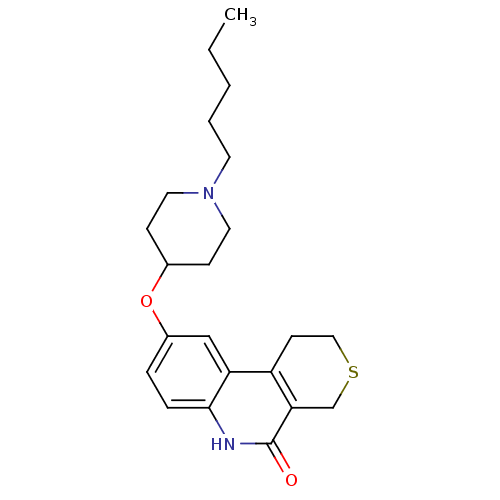
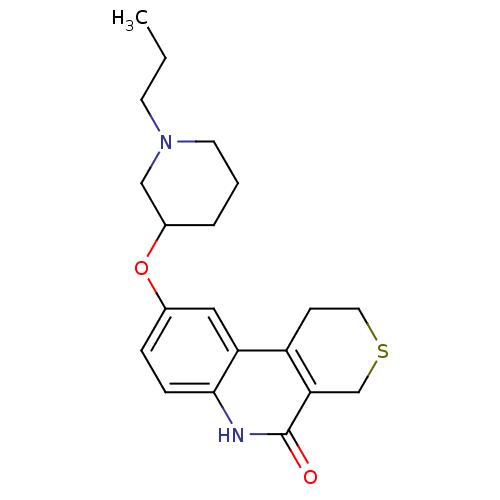
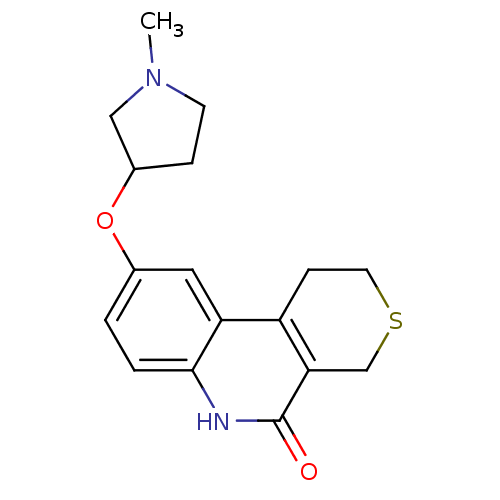
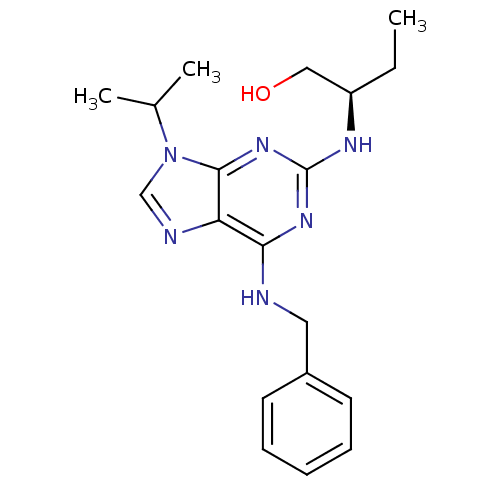
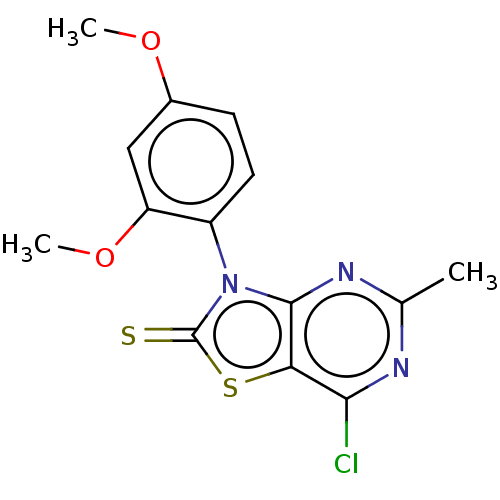
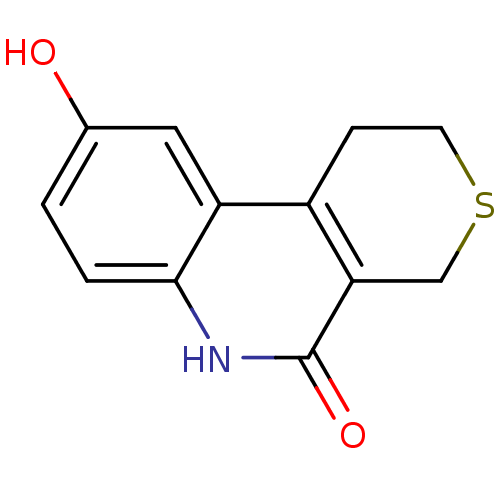
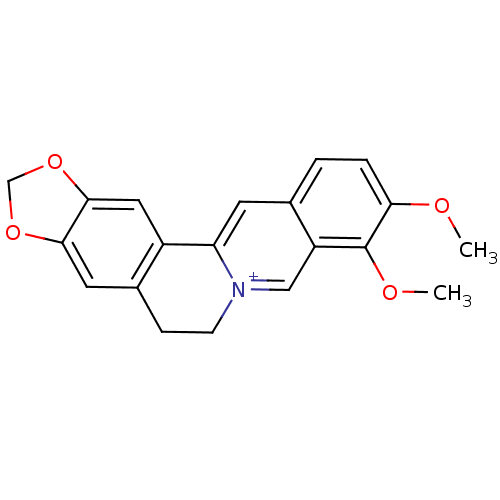
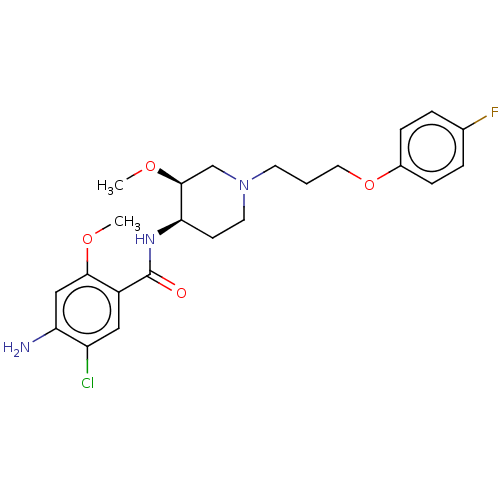
Affinity DataKi: 990nMAssay Description:Inhibition of BChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
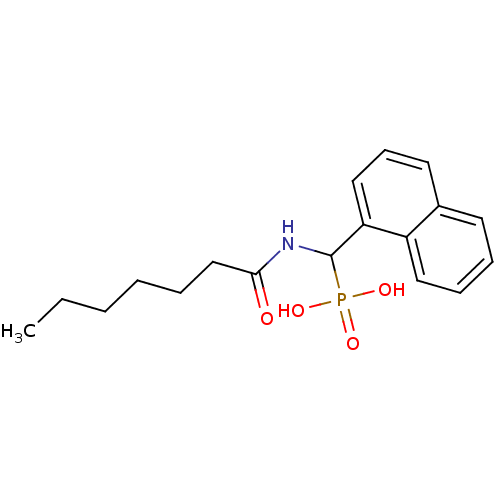
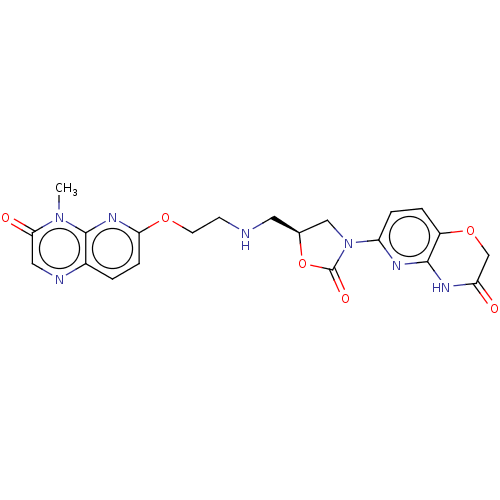
Affinity DataKi: 1.17E+3nMAssay Description:Inhibition of AChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
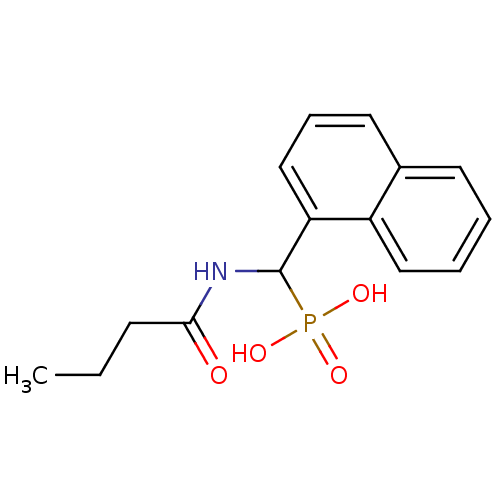
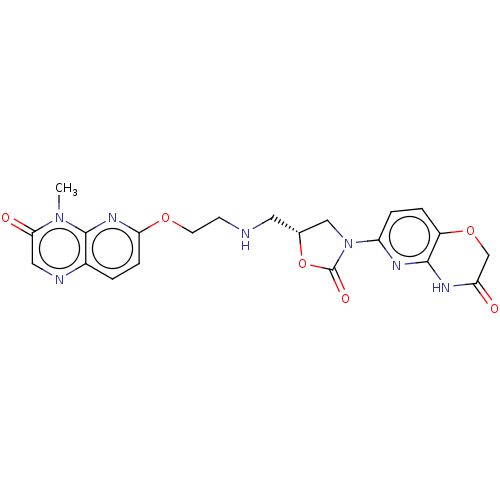
Affinity DataKi: 1.30E+3nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
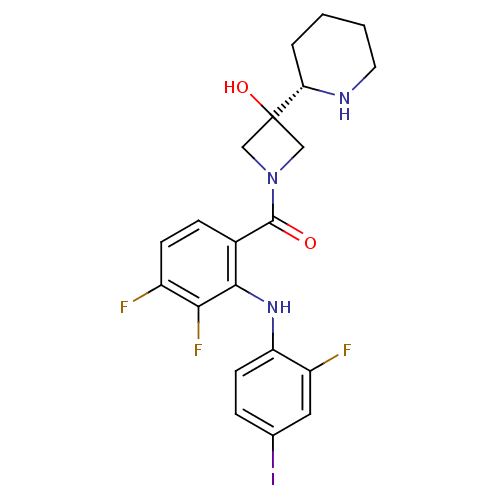
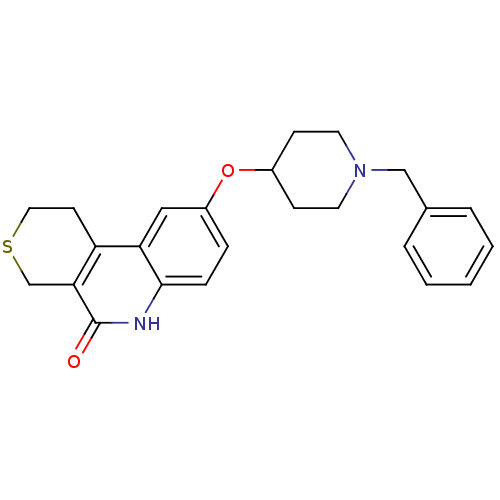
Affinity DataKi: 1.50E+3nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 2.49E+3nMAssay Description:Inhibition of AChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.78E+3nMAssay Description:Inhibition of BChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 4.88E+3nMAssay Description:Inhibition of BChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 5.00E+3nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 7.20E+3nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 7.97E+3nMAssay Description:Inhibition of AChE (unknown origin) by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.00E+3nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 1.10E+4nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
Affinity DataKi: 1.21E+4nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.39E+4nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.40E+4nMAssay Description:Noncompetitive inhibition of human recombinant BACE1 after 60 mins by Dixon plot analysisMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 3.10E+4nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 5.70E+4nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 1.02E+5nMpH: 4.9Assay Description:Uncompetitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometr...More data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 1.95E+5nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 2.22E+5nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 2.38E+5nMpH: 4.9Assay Description:Competitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometryMore data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 4.43E+5nMpH: 4.9Assay Description:Uncompetitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometr...More data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 4.46E+5nMpH: 4.9Assay Description:Uncompetitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometr...More data for this Ligand-Target Pair
TargetFe(3+)-Zn(2+) purple acid phosphatase(Phaseolus vulgaris)
The University Of Queensland
Curated by ChEMBL
The University Of Queensland
Curated by ChEMBL
Affinity DataKi: 6.54E+5nMpH: 4.9Assay Description:Uncompetitive inhibition of red kidney bean PAP using para-nitrophenol as substrate at pH 4.9 preincubated for 10 mins by UV-visible spectrophotometr...More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 1(Homo sapiens (Human))
Genentech
Curated by ChEMBL
Genentech
Curated by ChEMBL
Affinity DataIC50: 4.20nMAssay Description:Inhibition of MEK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
Affinity DataIC50: 47nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
Affinity DataIC50: 51nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
Affinity DataIC50: 67nMAssay Description:E. coli gyrase supercoiling and its inhibition was assayed using a kit procured from Inpiralis (K0001) and the protocol (PMID: 2172086) was adapted w...More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Egyptian Russian University
Curated by ChEMBL
Egyptian Russian University
Curated by ChEMBL
Affinity DataIC50: 82nMAssay Description:Inhibition of CDK1/cyclinB (unknown origin)More data for this Ligand-Target Pair
TargetCyclin-dependent kinase/G2/mitotic-specific cyclin- 1(Homo sapiens (Human))
Egyptian Russian University
Curated by ChEMBL
Egyptian Russian University
Curated by ChEMBL
Affinity DataIC50: 97nMAssay Description:Inhibition of CDK1/cyclinB (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of AChE (unknown origin) using acetylthiocholine as substrate assessed as substrate hydrolysis by spectrophotometric/Ellman methodMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Bugworks Research
US Patent
Bugworks Research
US Patent
Affinity DataIC50: 150nMAssay Description:Compounds were solubilised to 100 mM in DMSO before dilution in HBPS to 300 μM. 6-Point concentration-response curves were generated using 3.16-...More data for this Ligand-Target Pair

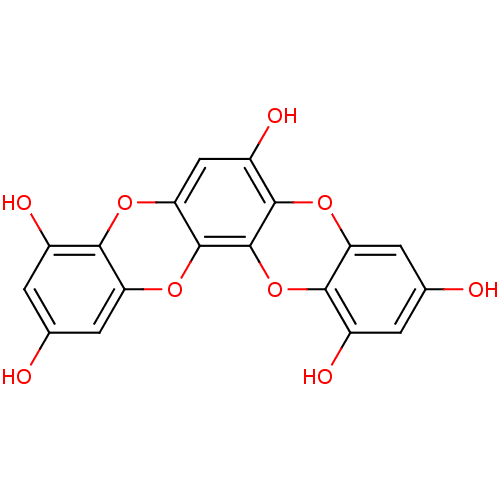
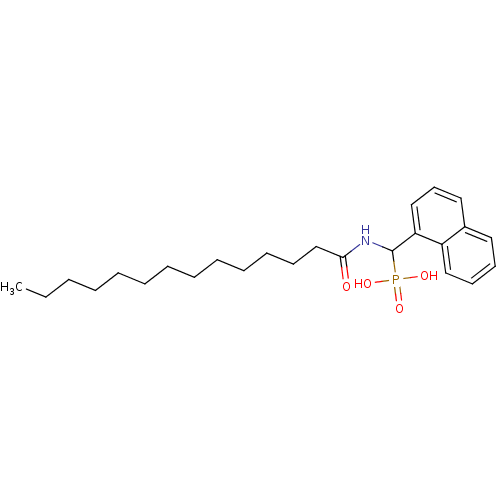

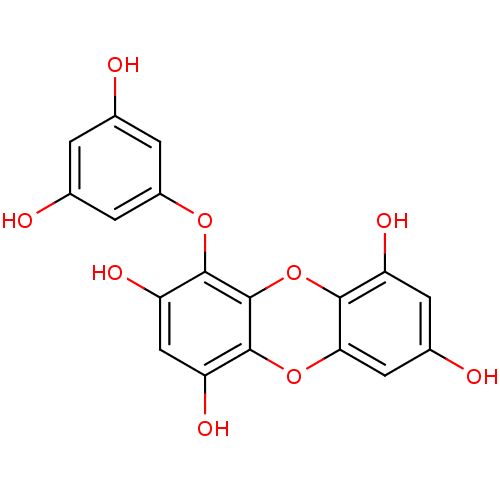
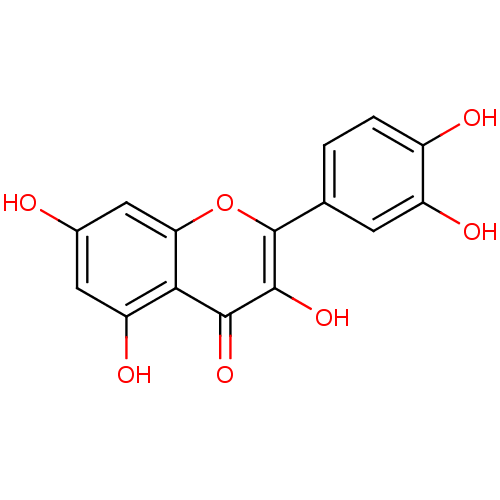

 3D Structure (crystal)
3D Structure (crystal)