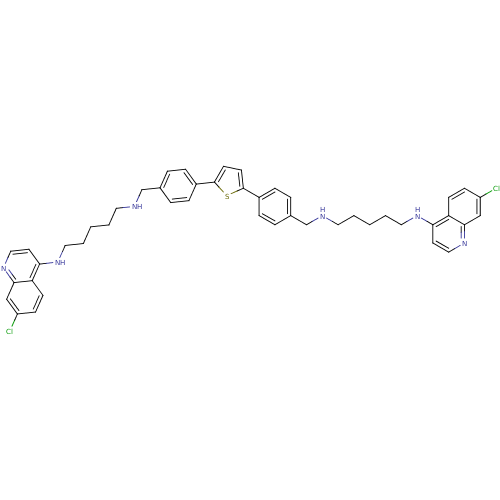
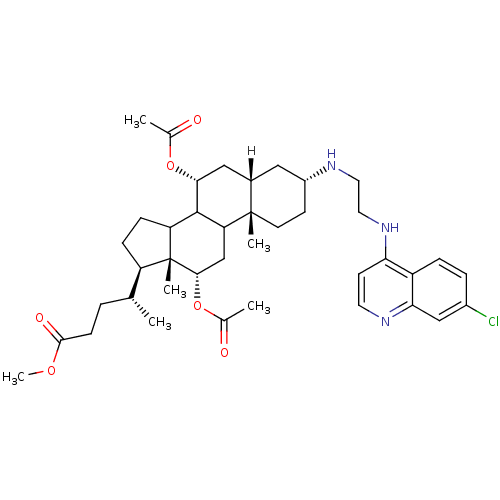
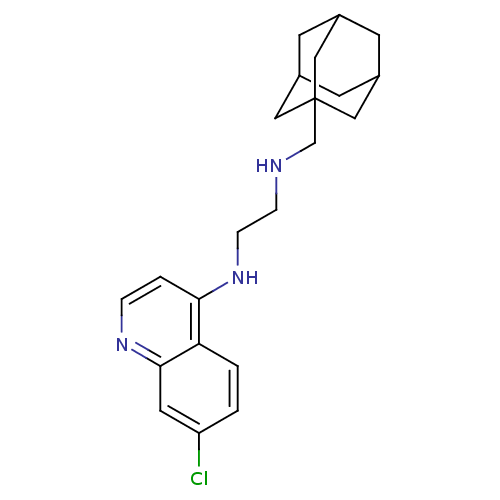
Affinity DataKi: 302nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 535nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 572nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 882nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 889nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.10E+3nMAssay Description:Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uMMore data for this Ligand-Target Pair
Affinity DataKi: 8.52E+3nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataKi: 8.70E+3nMAssay Description:Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uMMore data for this Ligand-Target Pair
Affinity DataKi: 1.09E+4nMAssay Description:Competitive inhibition of clostridium botulinum Botulinum neurotoxin type A light chain by RP-HPLC analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of Clostridium botulinum BoNT/A light chainMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.18E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 1.89E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 3.20E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair
Affinity DataIC50: 5.01E+4nMpH: 7.3 T: 2°CAssay Description:Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA...More data for this Ligand-Target Pair