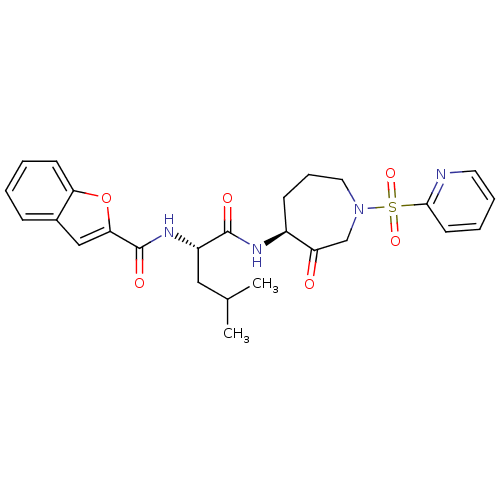
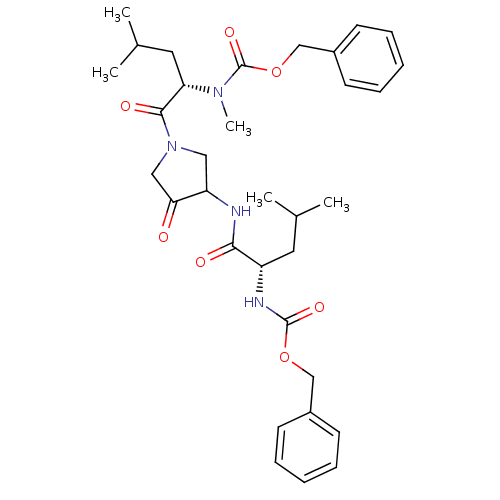
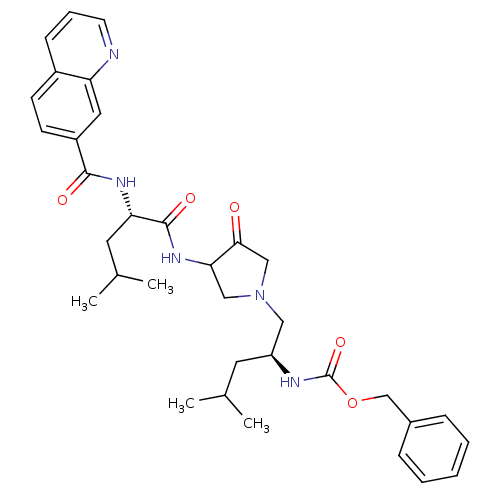
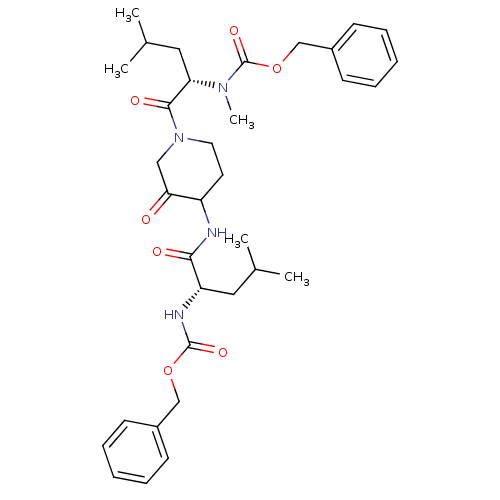
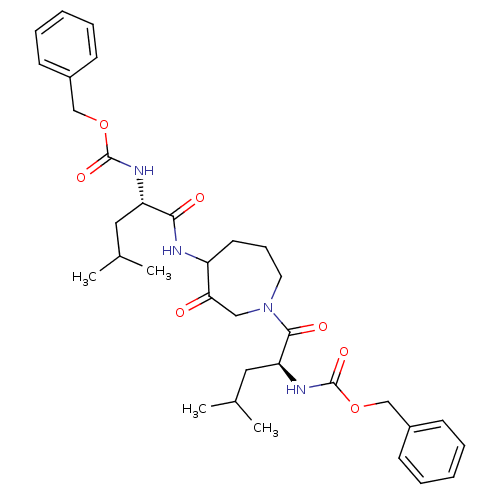
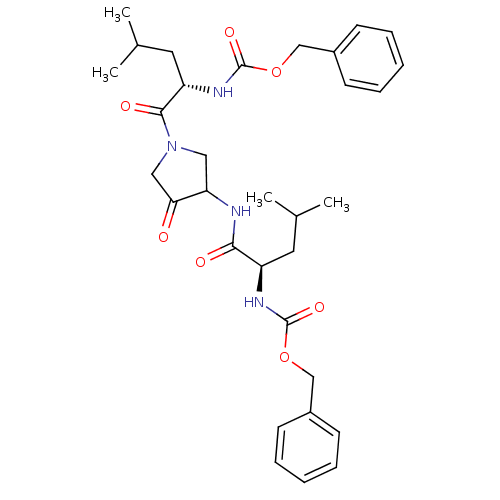
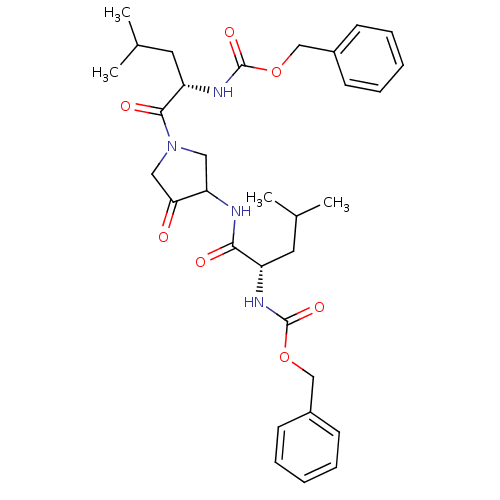
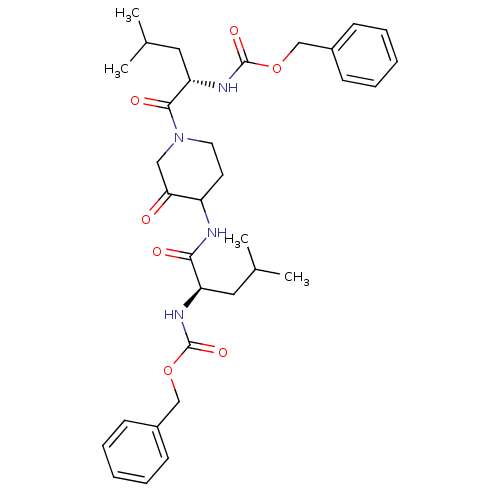
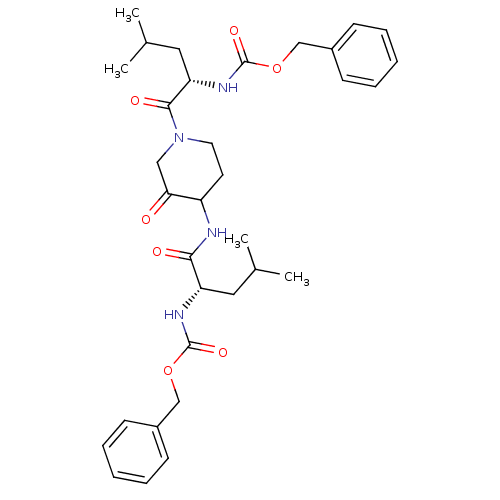
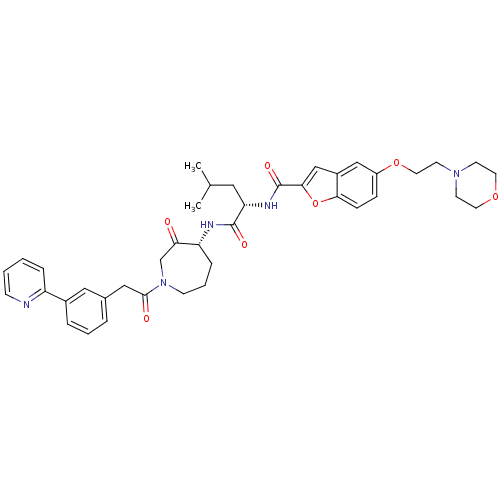
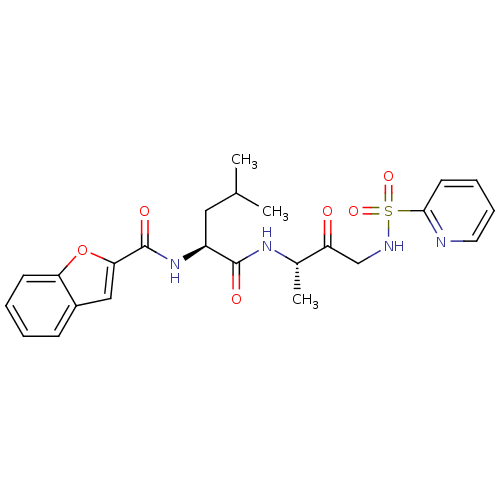
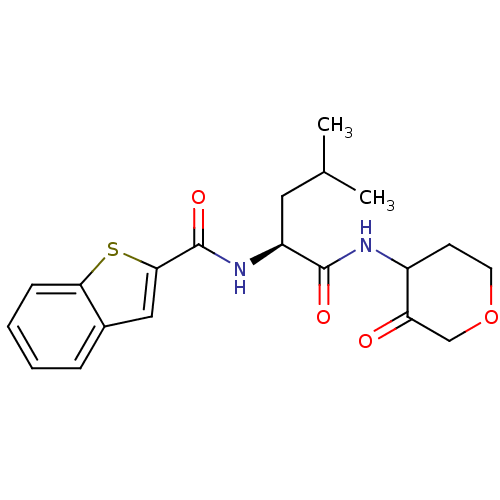
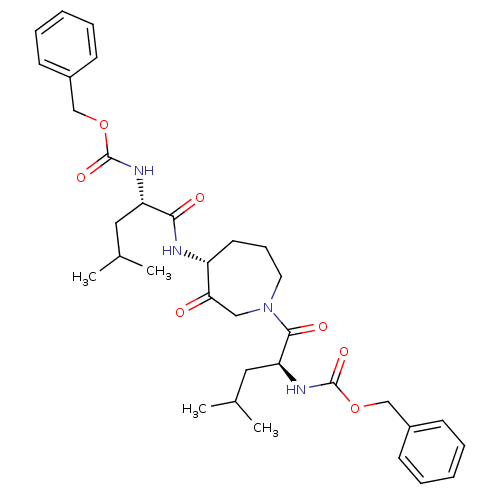
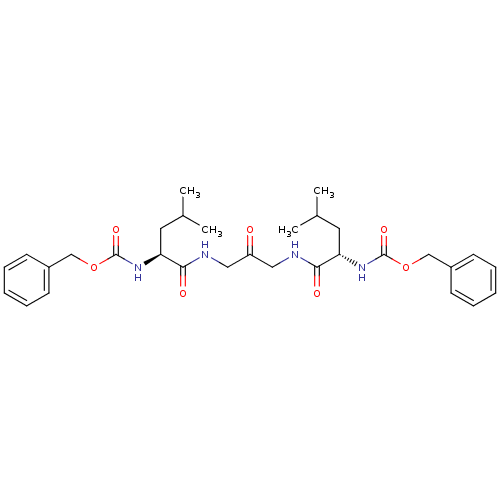
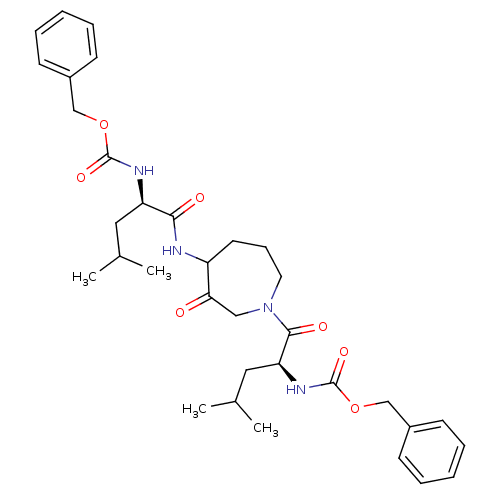
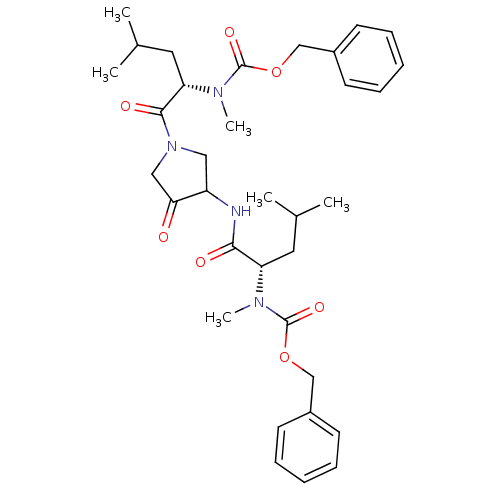
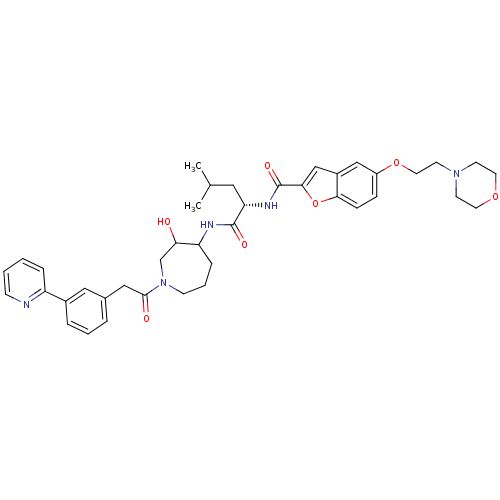
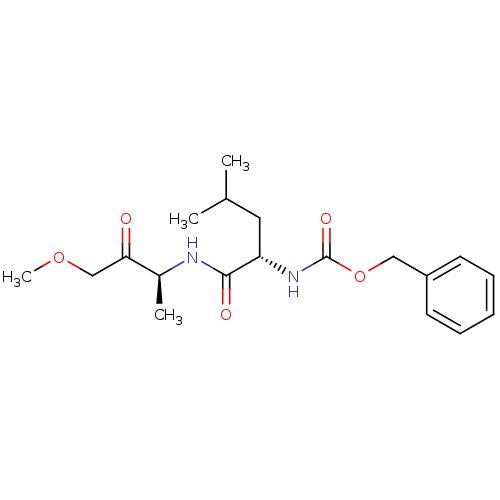
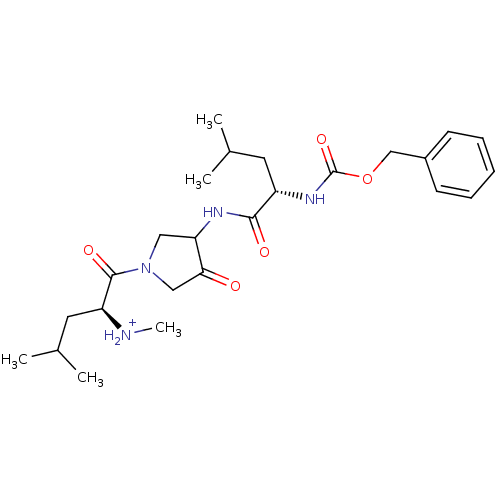
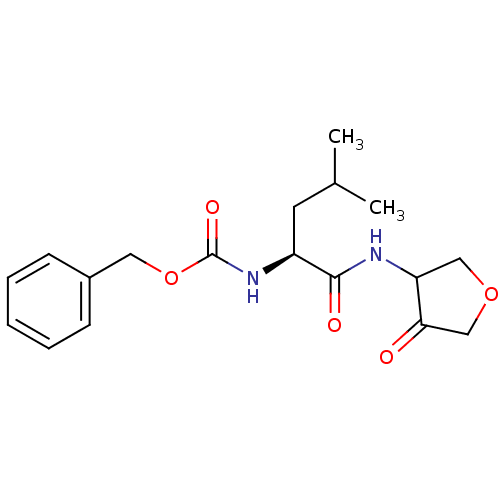
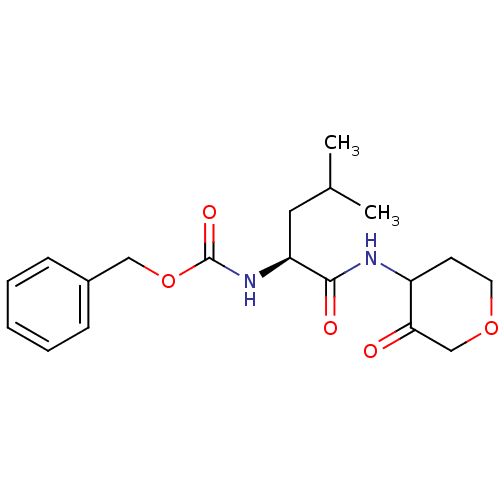
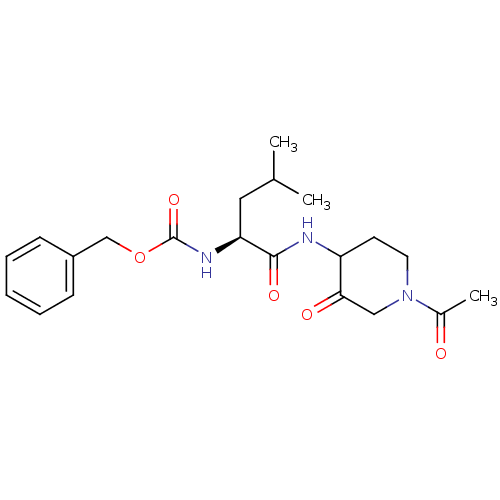
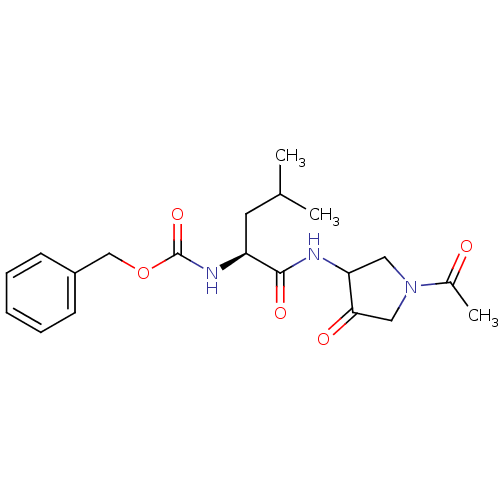
Affinity DataKi: 0.00480nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.490nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.1kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 1.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.60nM ΔG°: -49.7kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 1.90nM ΔG°: -49.3kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.30nM ΔG°: -48.8kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 2.60nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 2.60nM ΔG°: -48.5kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4.70nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 4.80nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 7.5nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 8nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 11nM ΔG°: -45.0kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 15nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 20nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 22nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 26nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 30nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 39nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 44nM ΔG°: -41.6kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 46nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 51nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 54nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 59nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 60nM ΔG°: -40.8kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibitory activity of the compound against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 77nM ΔG°: -40.2kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 97nMAssay Description:Inhibitory activity of the compound against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Inhibitory activity of the compound against Human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 140nM ΔG°: -38.7kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 150nM ΔG°: -38.6kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 180nM ΔG°: -38.1kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 230nM ΔG°: -37.5kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 250nM ΔG°: -37.3kJ/molepH: 5.5 T: 2°CAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 250nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Inhibitory activity of the compound against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 290nMAssay Description:Assays were carried out in the presence of variable concentrations of test compound. Reactions were initiated by addition of enzyme to buffered solut...More data for this Ligand-Target Pair

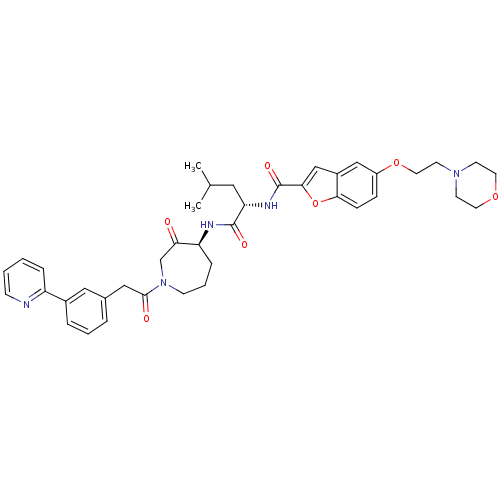
 3D Structure (crystal)
3D Structure (crystal)