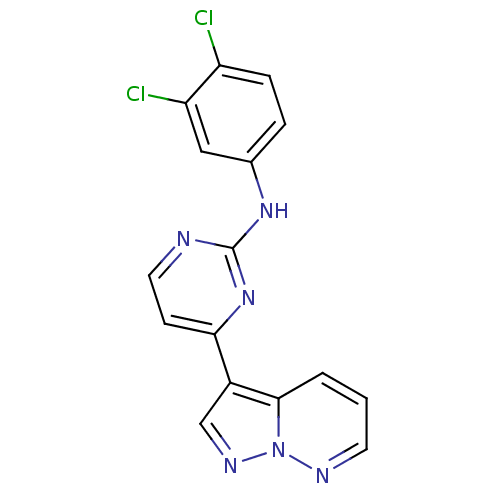
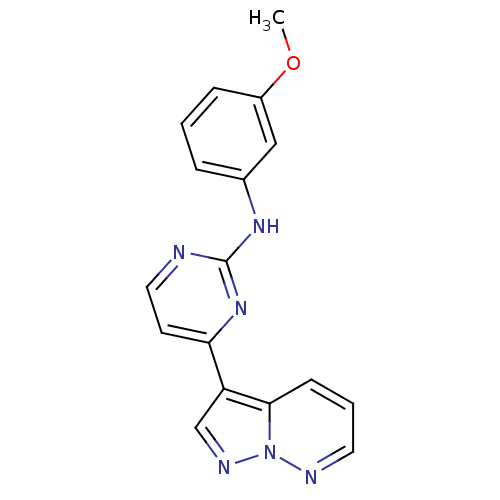
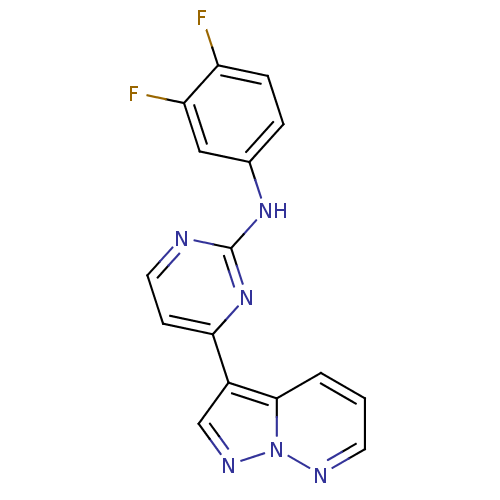
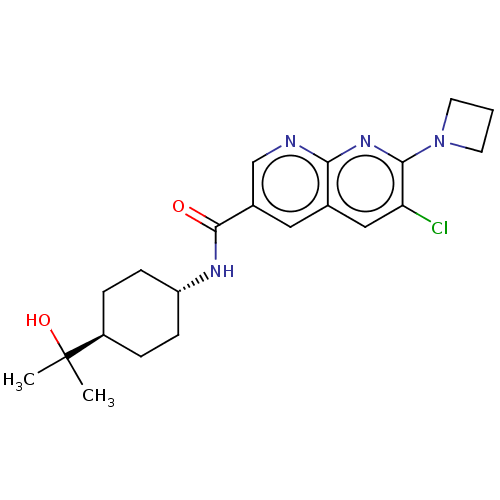
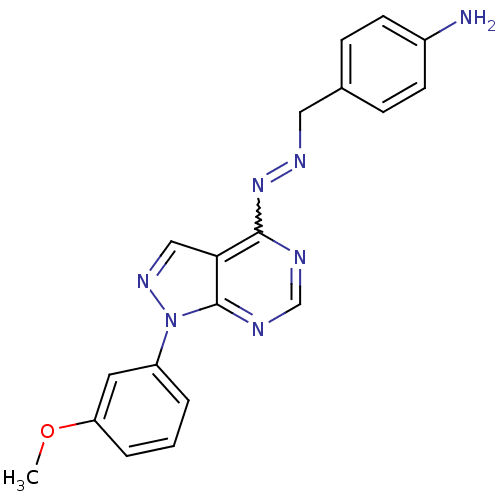
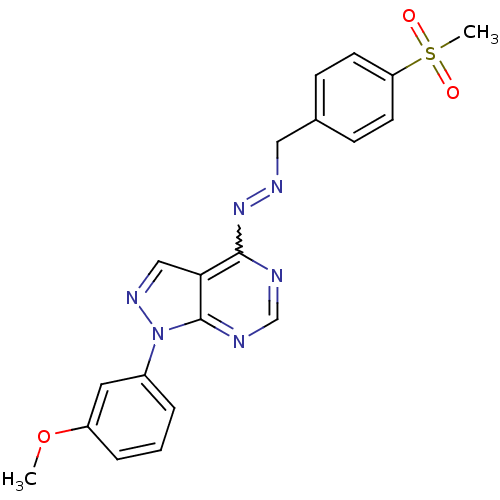
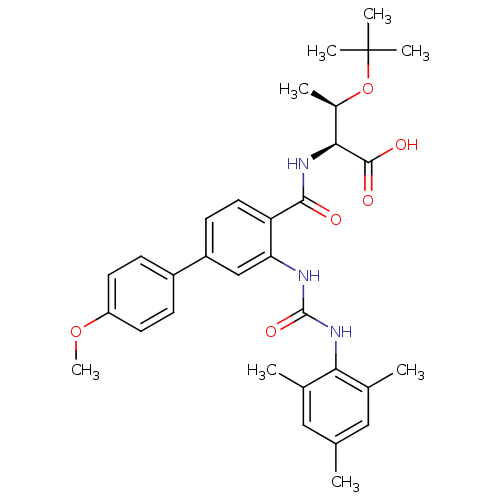
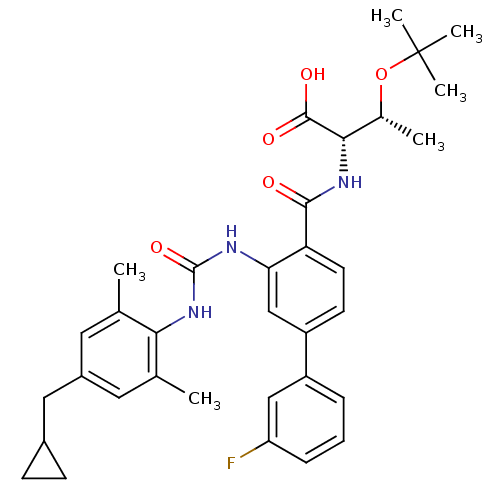
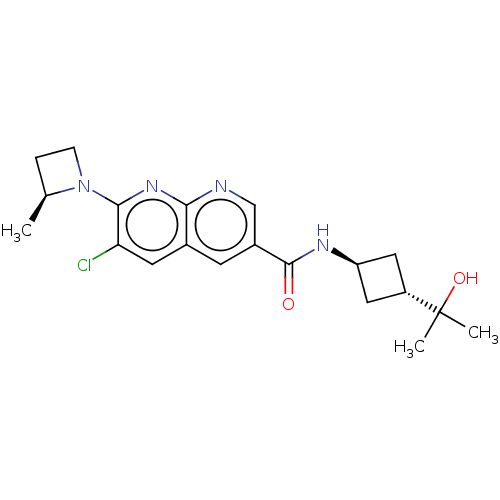
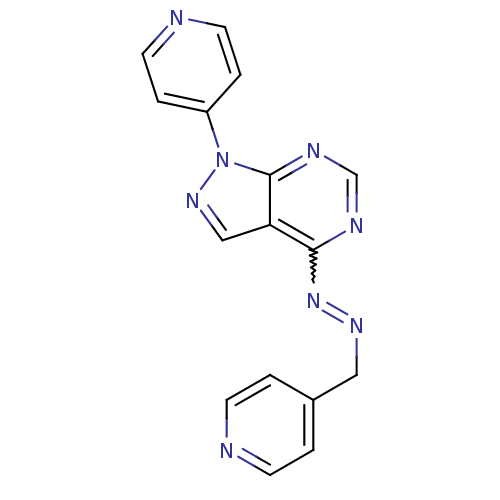
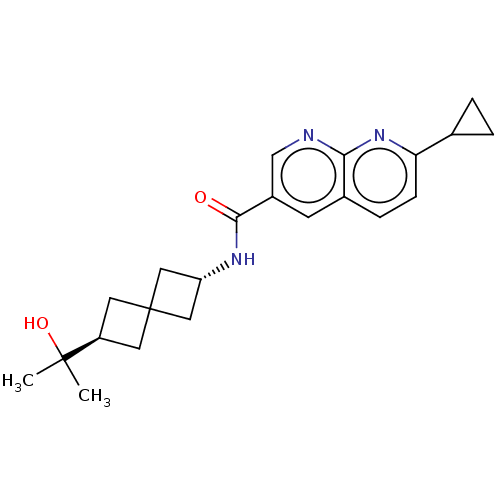
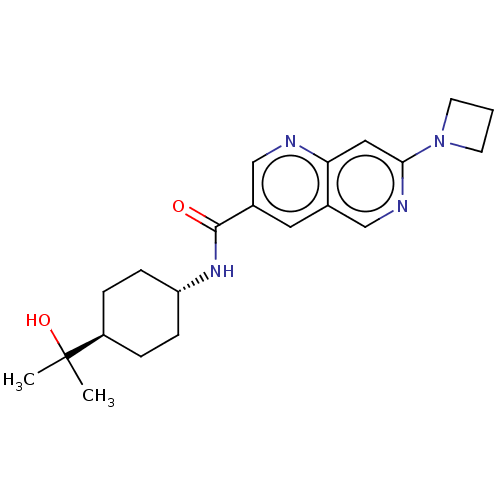
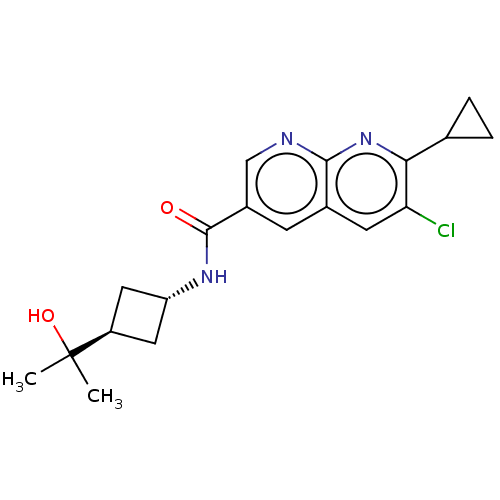
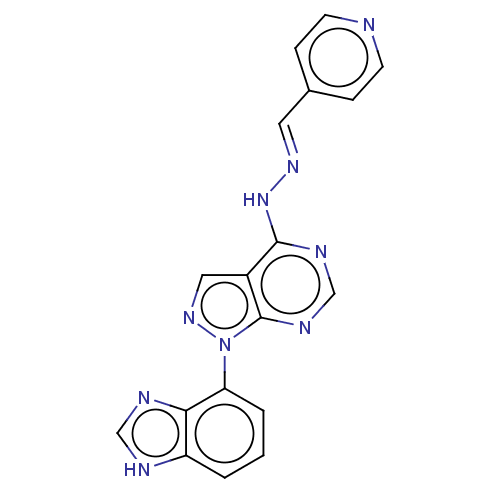
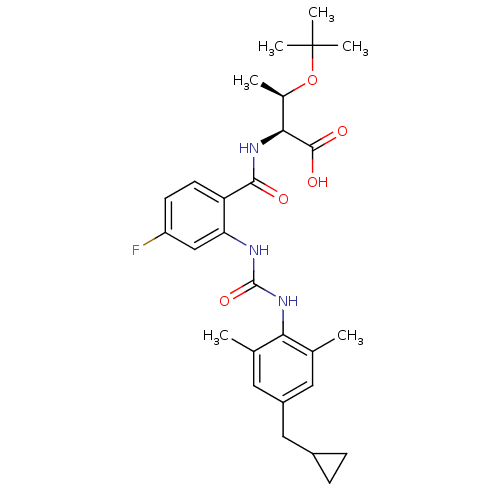
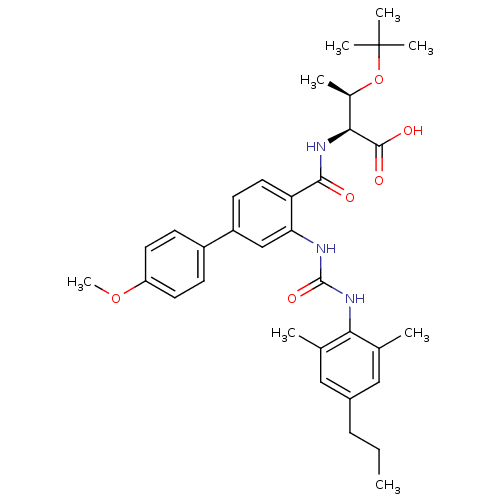
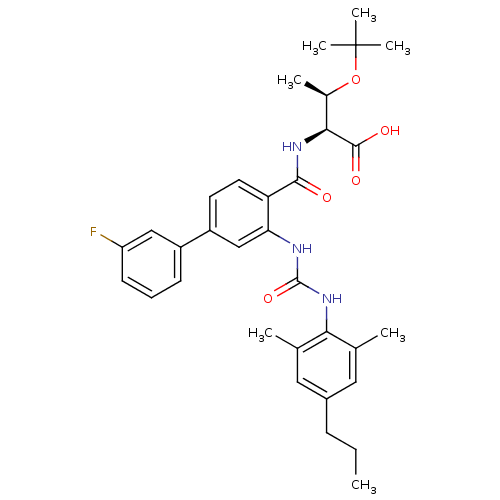
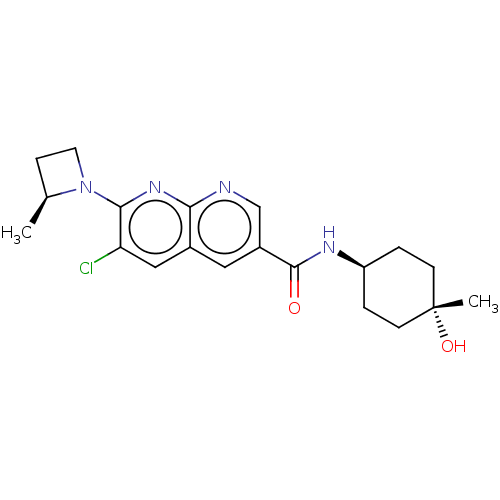
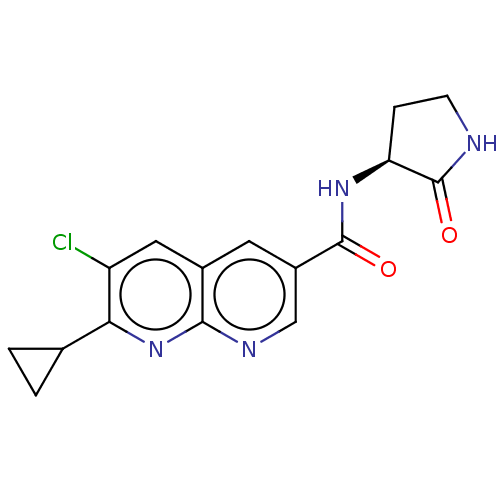
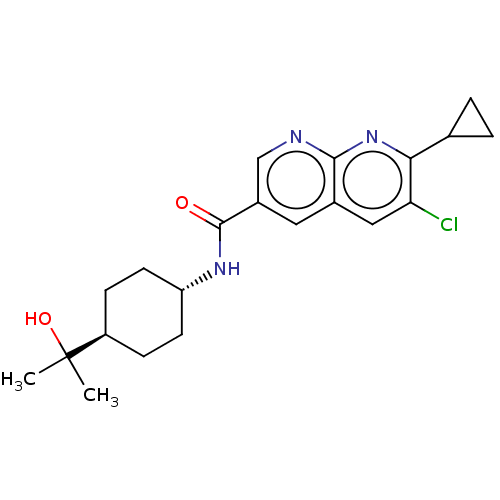
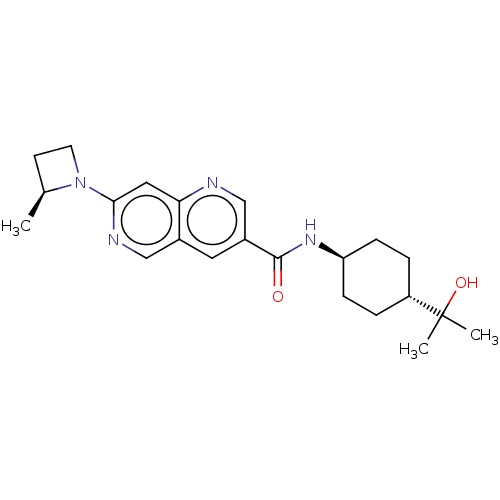
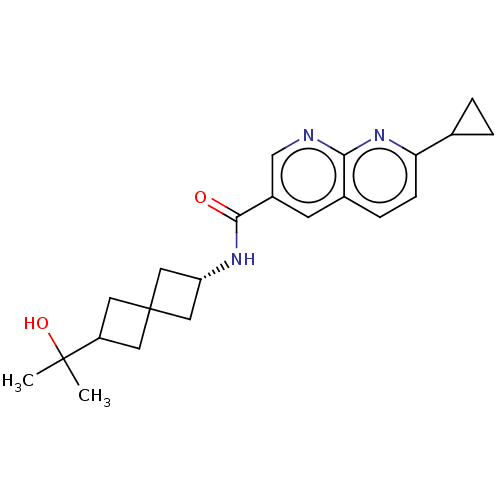
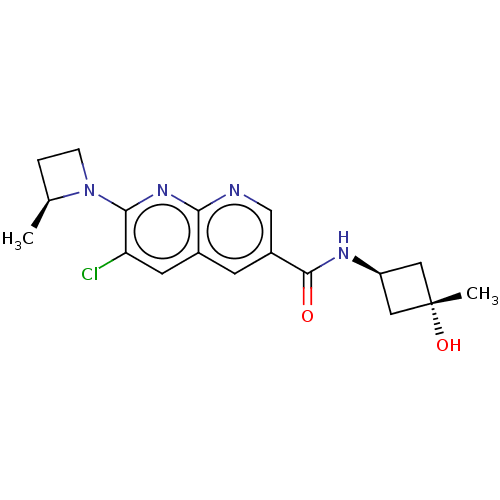
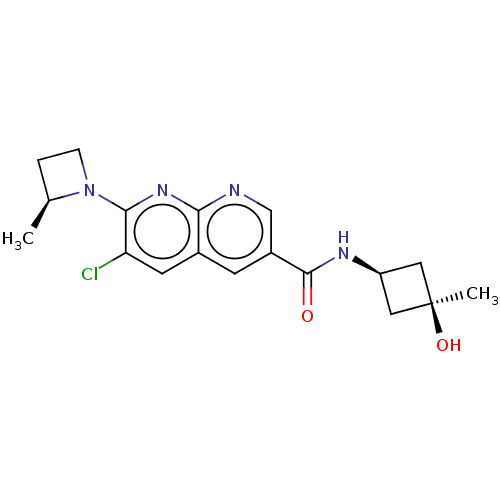
Affinity DataKi: 0.300nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 1nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 1nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 1nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 1nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataKi: 2nM IC50: <10nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Michigan
Curated by ChEMBL
University Of Michigan
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Displacement of phenylethanolamine from human PNMT by competitive inhibition assayMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Michigan
Curated by ChEMBL
University Of Michigan
Curated by ChEMBL
Affinity DataKi: 50nMAssay Description:Binding affinity to wild type human PNMT by liquid scintillation spectrometry in presence of [3H]AdoMetMore data for this Ligand-Target Pair
TargetPhenylethanolamine N-methyltransferase(Homo sapiens (Human))
University Of Michigan
Curated by ChEMBL
University Of Michigan
Curated by ChEMBL
Affinity DataKi: 120nMAssay Description:Binding affinity to wild type human PNMT by liquid scintillation spectrometry in presence of [3H]AdoMetMore data for this Ligand-Target Pair
Affinity DataIC50: 1nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.2 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 1nMpH: 7.2 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily A member 3(Homo sapiens (Human))
Glaxosmithkline
Curated by ChEMBL
Glaxosmithkline
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human voltage-gated potassium channel Kv1.3 expressed in L929 cells by whole-cell patch clamp assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 2nMpH: 7.2 T: 2°CAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 2.5nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 2.51nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-32P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.10nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.16nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.80nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand InfoPDB
Affinity DataIC50: 3.90nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 3.98nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.98nMAssay Description:The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 2.5 uM ATP/ [gamma-33P] ATP. Af...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 4nMpH: 7.6 T: 2°CAssay Description:An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t...More data for this Ligand-Target Pair
Affinity DataIC50: 4.40nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.60nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.70nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.80nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 4.90nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 5nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 5nMAssay Description:Inhibition of human H-PGDS expressed in Escherichia coli BL21 DE3 cells assessed as reduction in PGD2 production using PGH2 as substrate by RapidFire...More data for this Ligand-Target Pair
Ligand Info
Affinity DataIC50: 5nMAssay Description:Inhibition of glycogen synthase kinase-3 (GSK3-beta)More data for this Ligand-Target Pair

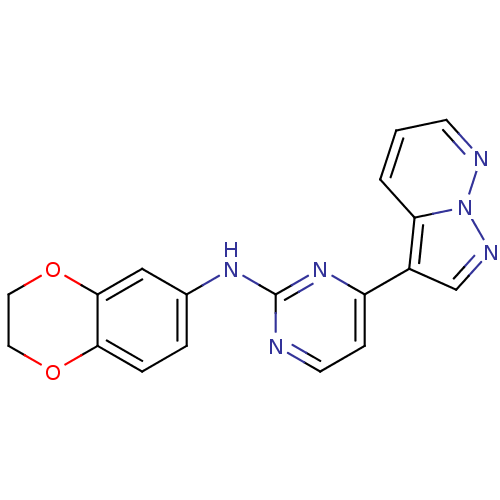
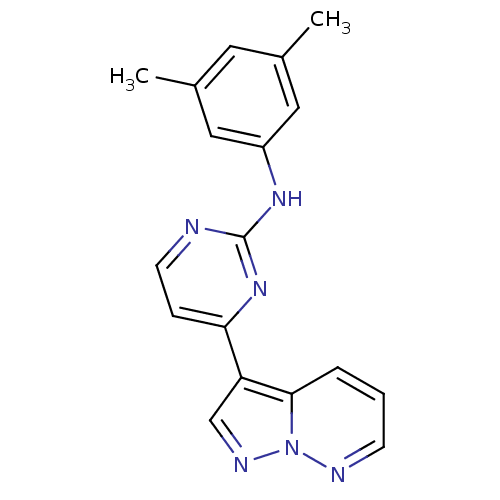
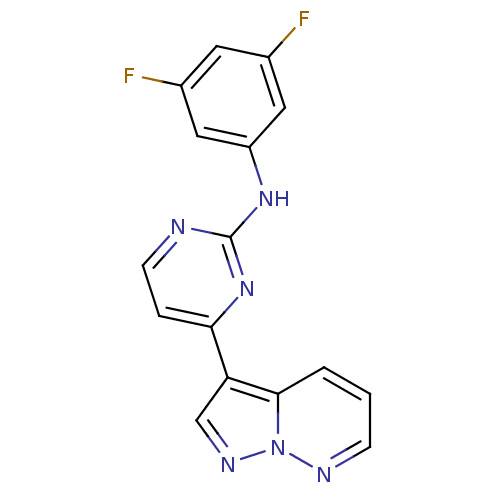
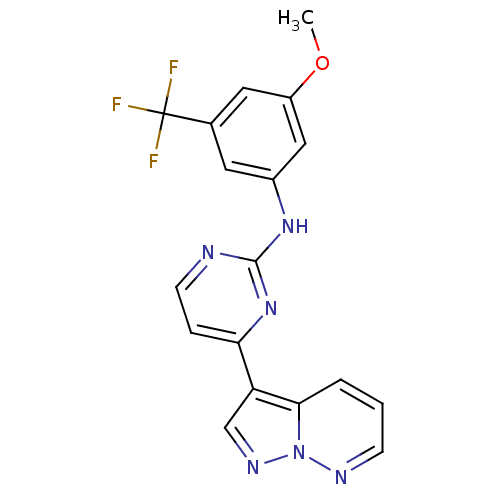
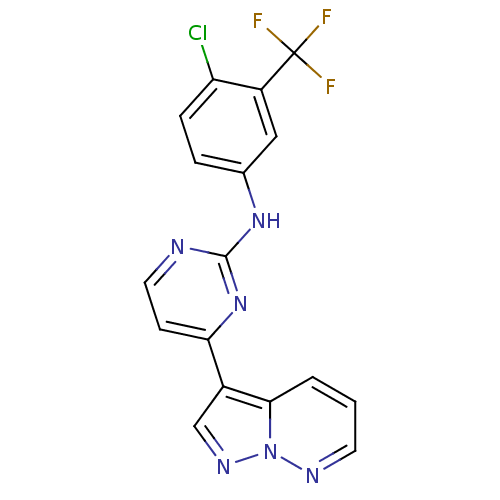
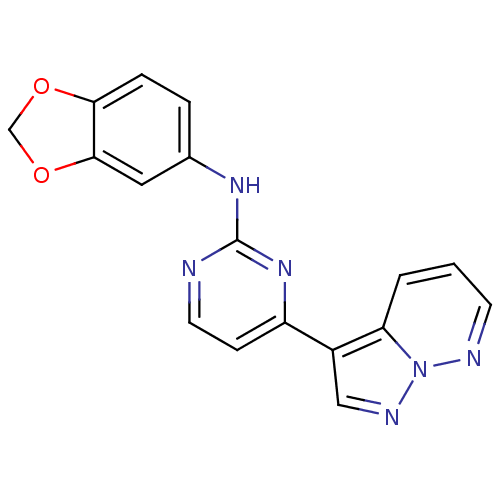
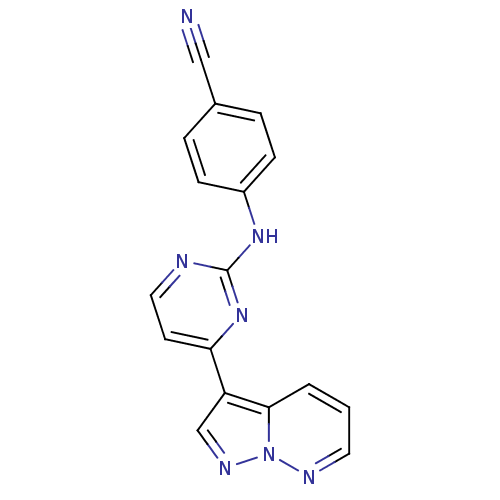
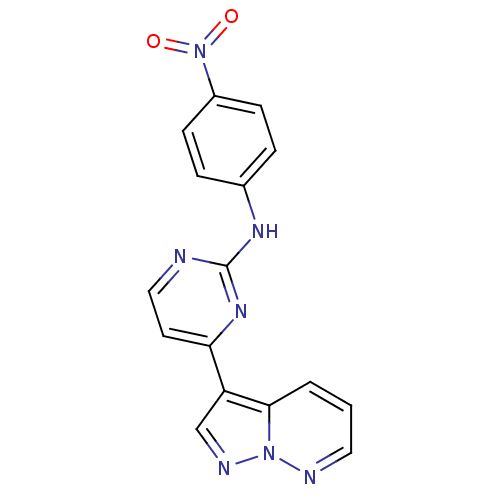
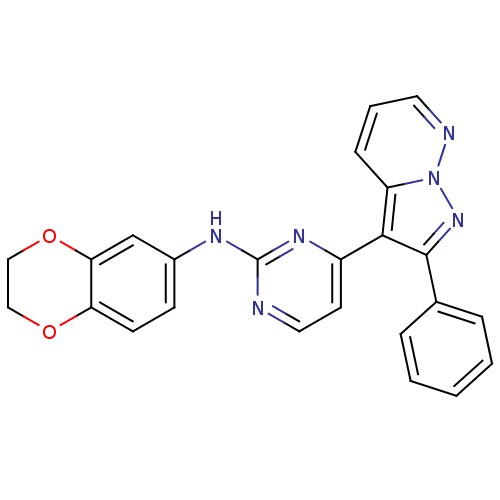
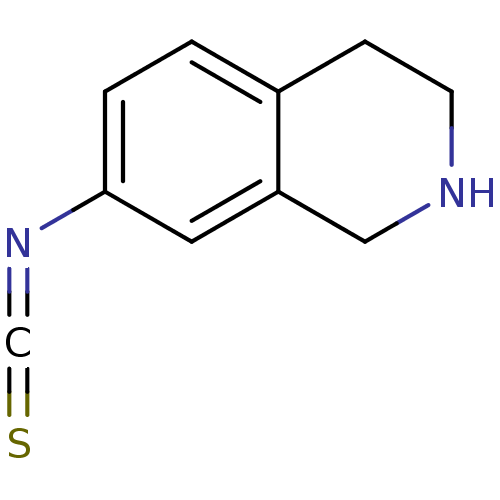
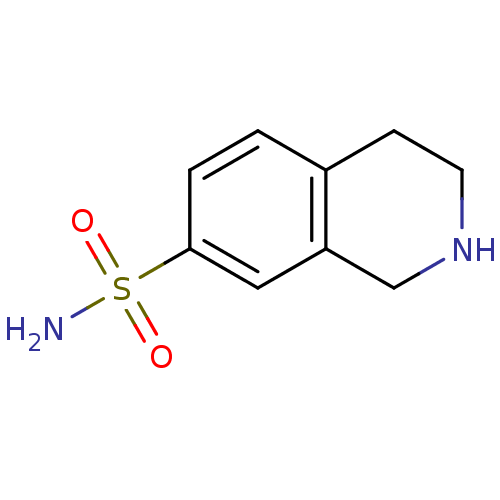
 3D Structure (crystal)
3D Structure (crystal)