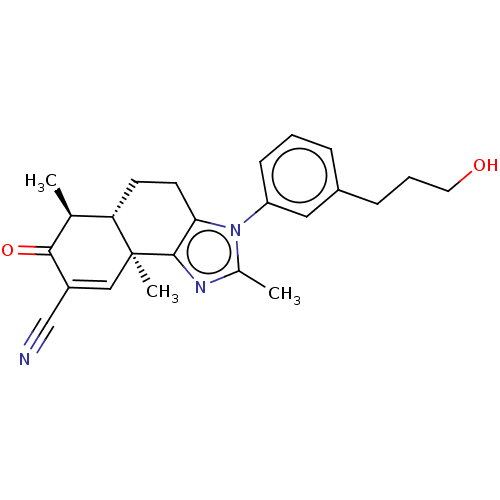
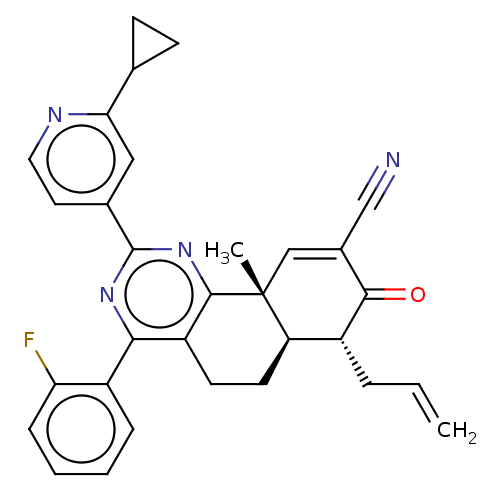
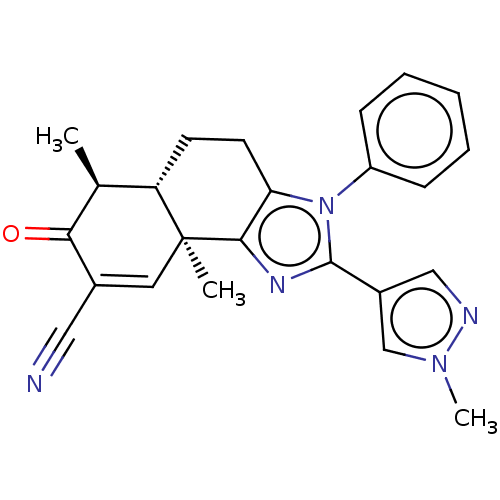
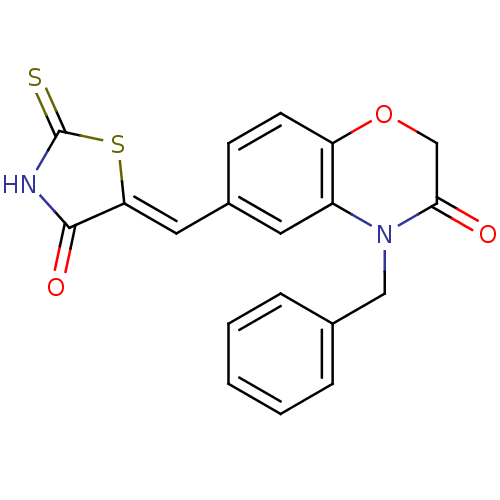
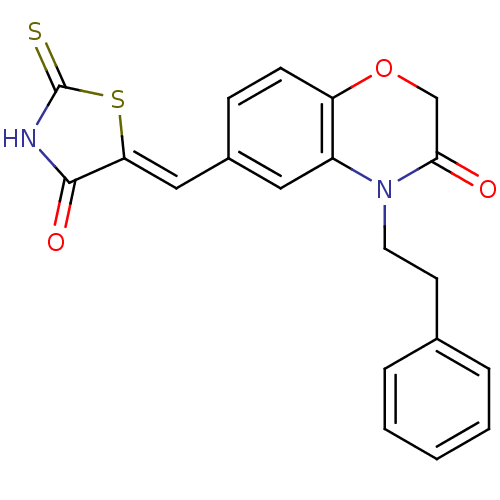
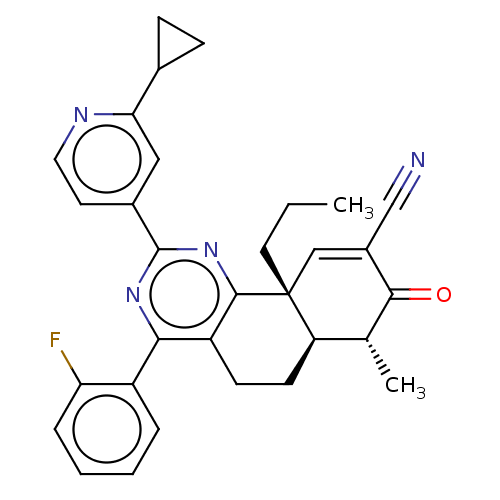
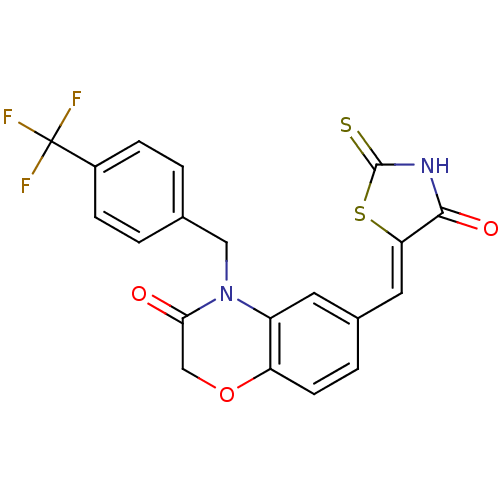
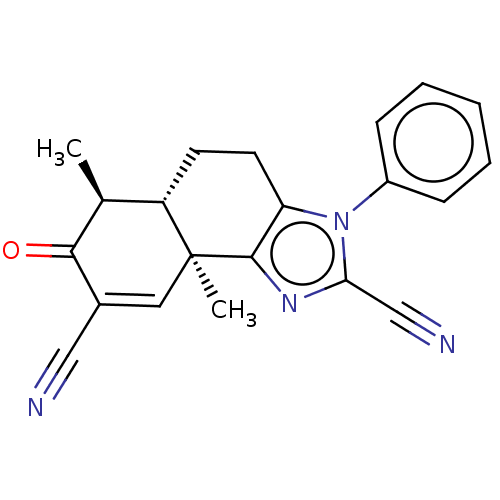
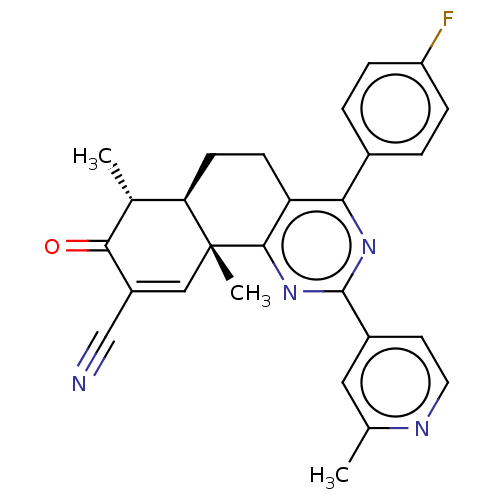
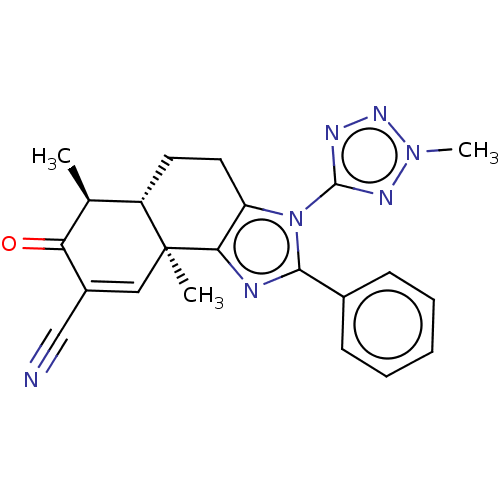
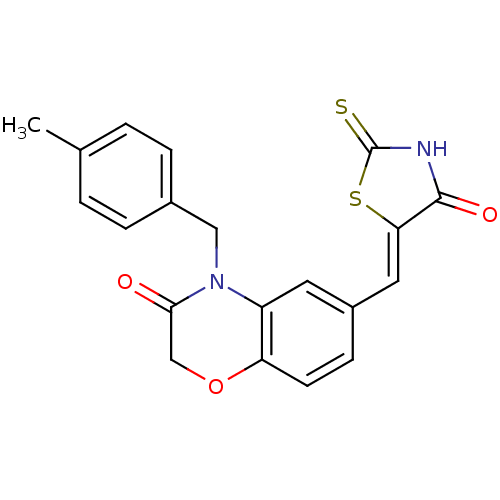
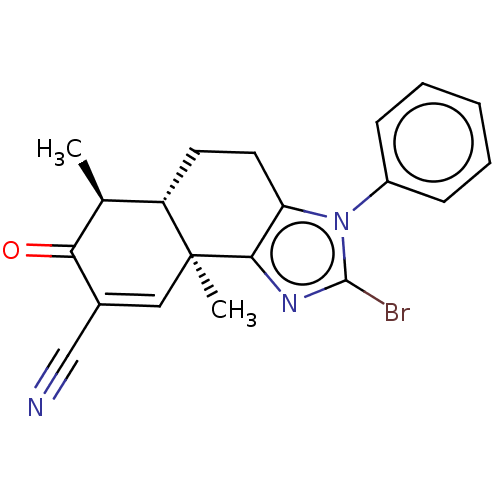
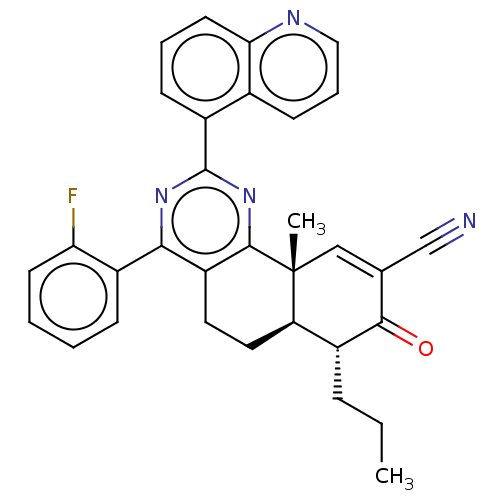
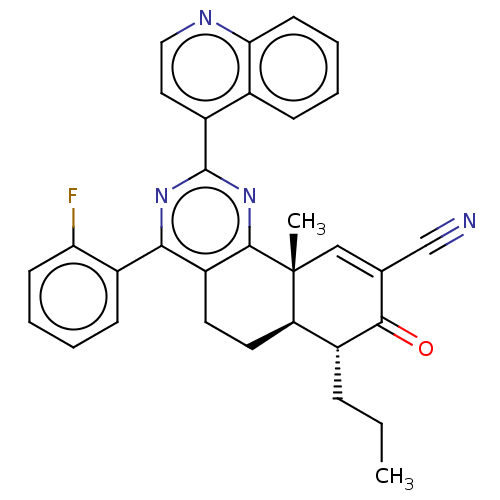
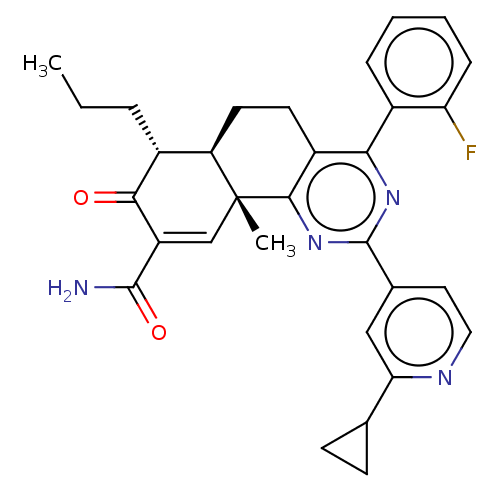
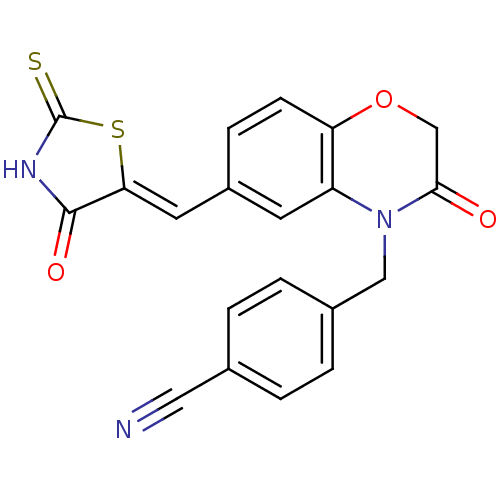
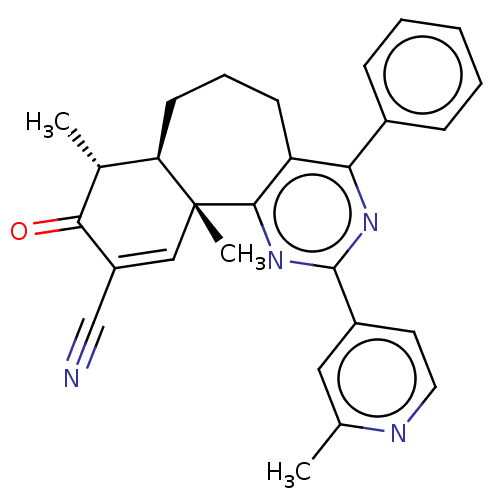
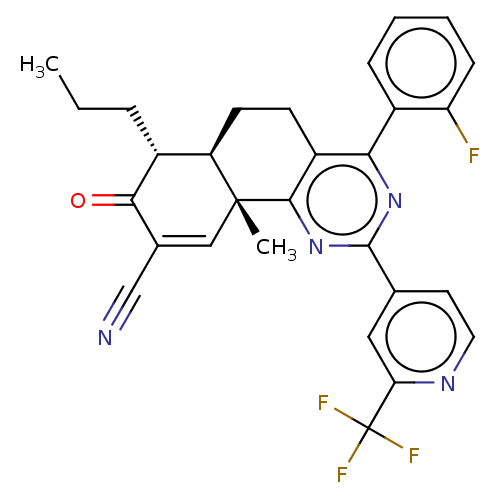
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 1.92nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
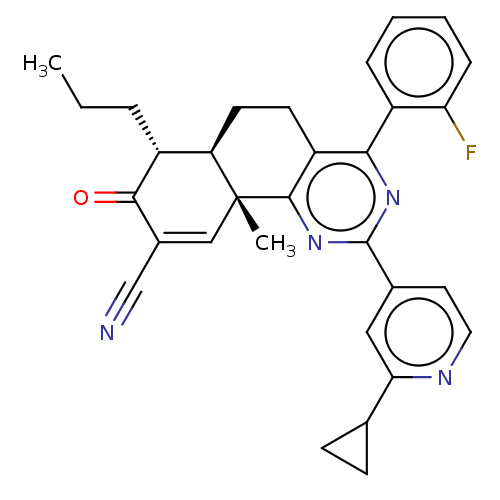
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.34nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
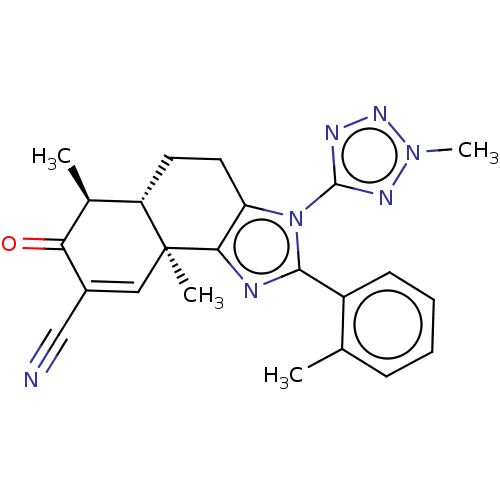
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.42nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
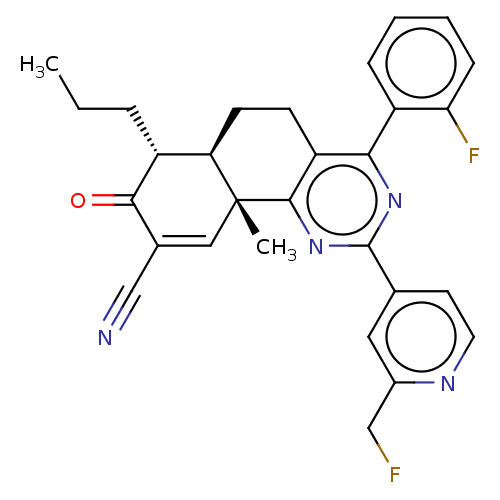
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.59nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.65nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.66nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 2.79nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.27nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.28nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.36nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.76nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.84nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 4.96nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 6.57nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 7.16nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 8.83nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 11.3nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
Affinity DataIC50: 15.4nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 19nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 19.4nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
Affinity DataIC50: 22.7nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 23nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 24.5nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 27.5nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 27.7nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as inhibition of T cell to Th17 differentiation by flow cytometryMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inverse agonist activity at full-length human RORgammat C285S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 28.8nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 29.5nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inverse agonist activity at human full-length RORgammaT expressed in Jurkat cells co-transfected with 5 copies of ROR response element after 18 hrs b...More data for this Ligand-Target Pair
Affinity DataIC50: 31.1nMAssay Description:RAW 264.7 cells were plated 1 day in advance of experiment at a concentration of 80,000 cells/well onto CellBIND® 96 well plates (Corning, N.Y.) in a...More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 33.2nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inverse agonist activity at full-length human RORgammat C345S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 35nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ...More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inverse agonist activity at RORgammat in human CD4-positive T cells assessed as suppression of T cell differentiation to Th17 cells by reduction in m...More data for this Ligand-Target Pair
TargetPhosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform/Phosphoinositide 3-kinase regulatory subunit 5(Homo sapiens (Human))
Pfizer
Pfizer
Affinity DataIC50: 36.1nMpH: 7.4 T: 2°CAssay Description:PI3Kgamma activity was assessed by incubation of baculoviral co-expressed regulatory and catalytic subunits (p101 and p110) with lipid micelles prepa...More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:The RORγ assay system was purchased from Indigo Biosciences. This nuclear receptor assay utilizes a human cell line that has been engineered to ...More data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inverse agonist activity at full-length human RORgammat C345S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inverse agonist activity at full-length human RORgammat C320S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 37nMAssay Description:Inverse agonist activity at full-length human RORgammat C455S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inverse agonist activity at wild type full-length human RORgammat co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inverse agonist activity at full-length human RORgammat C285S mutant co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inverse agonist activity at wild type full-length human RORgammat co-transfected with ROR response element by luciferase based reporter gene assayMore data for this Ligand-Target Pair

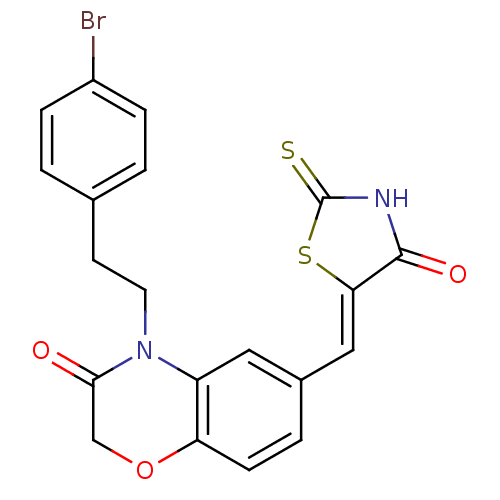
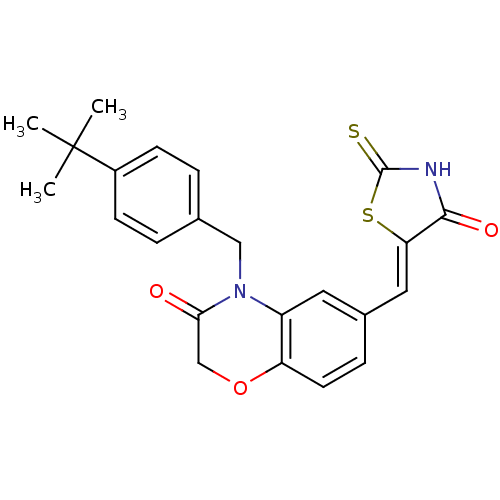
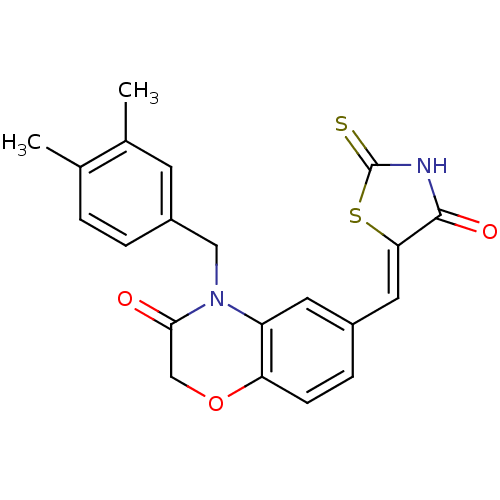
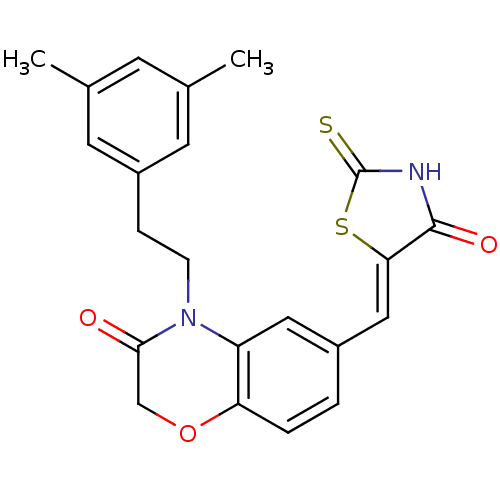
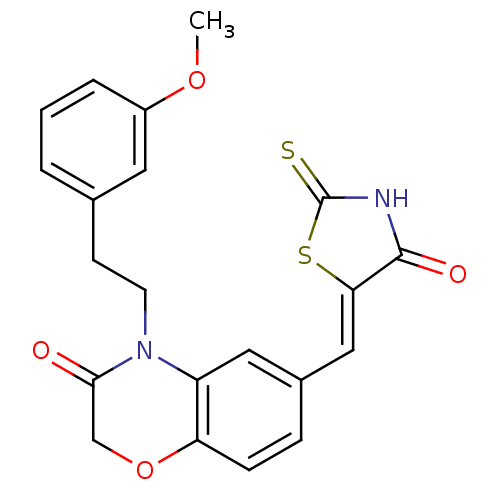
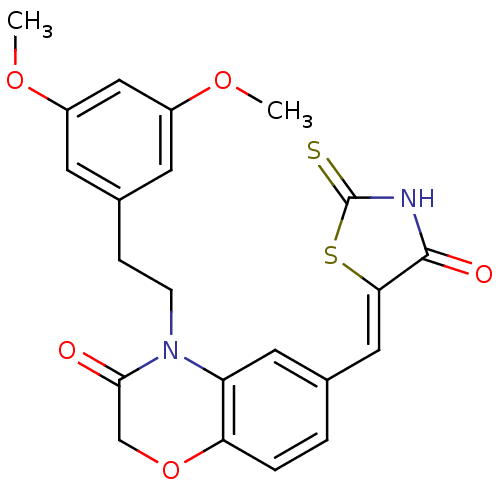
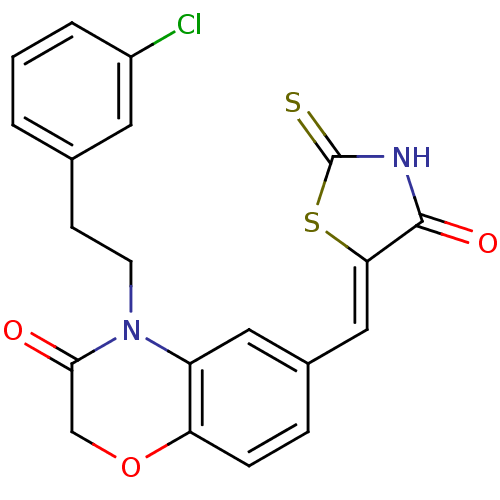
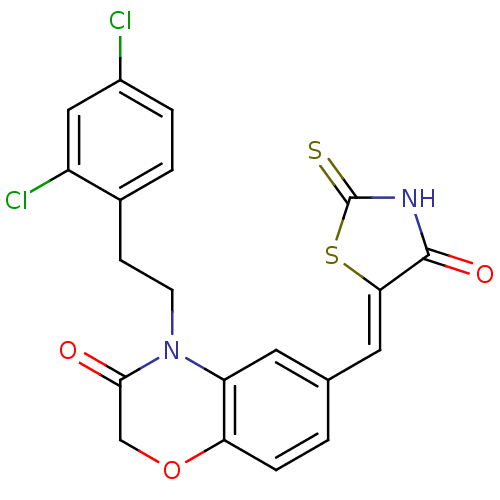
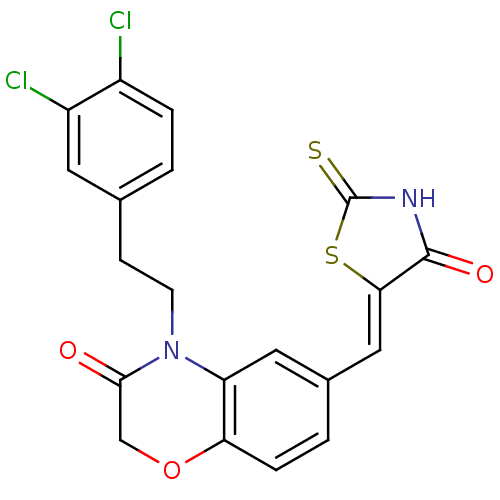
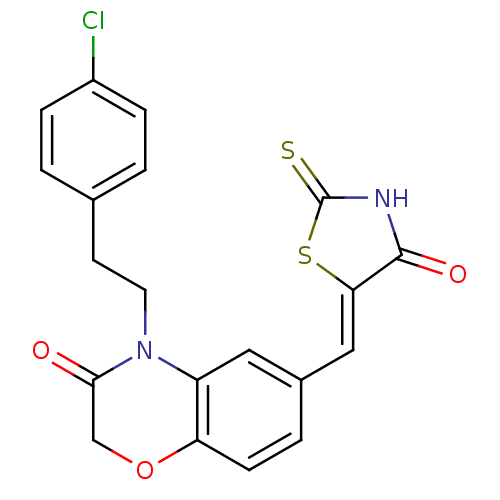
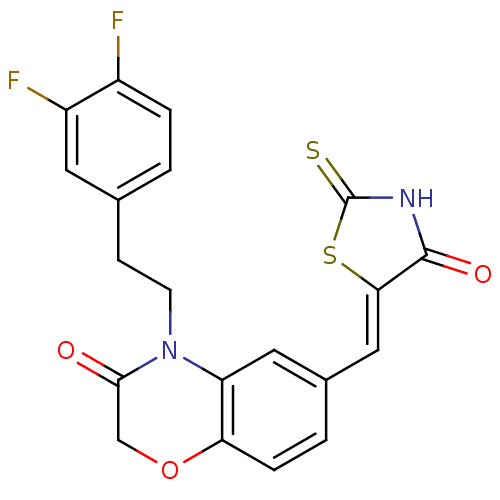
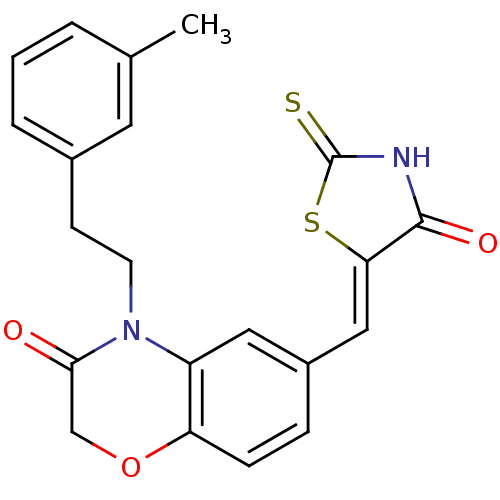
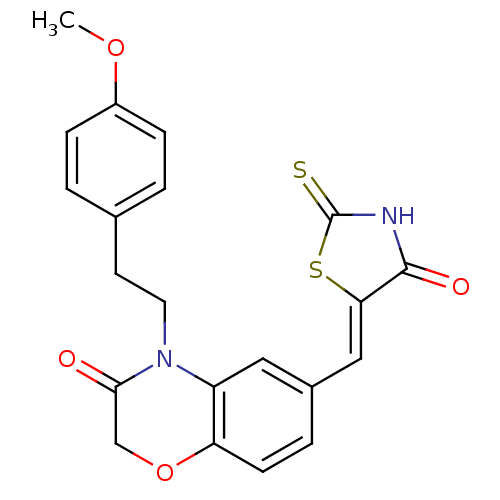
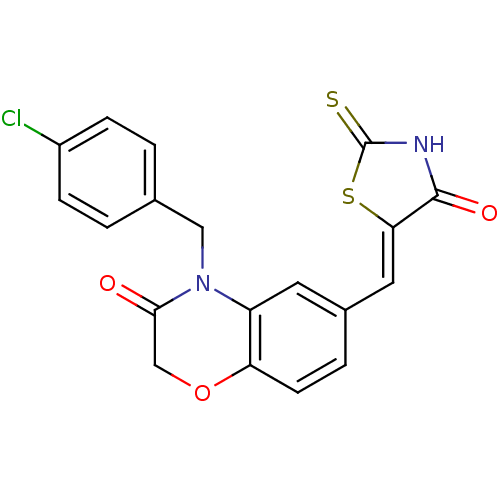
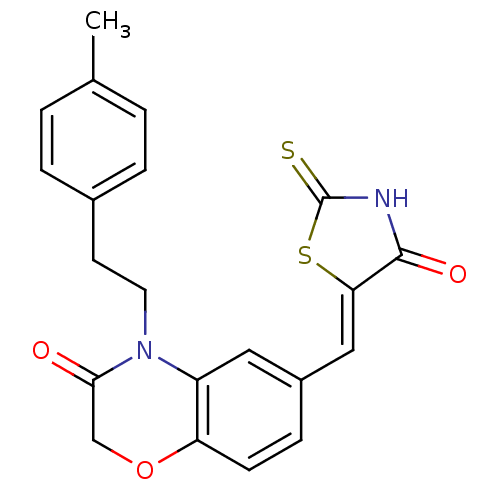
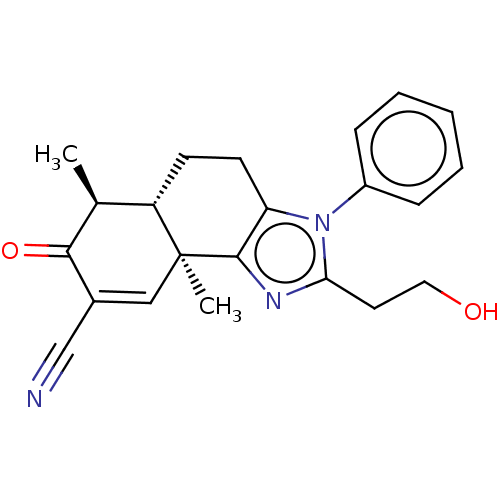
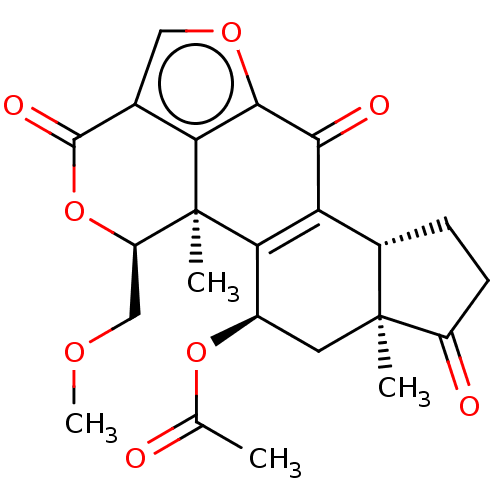
 3D Structure (crystal)
3D Structure (crystal)