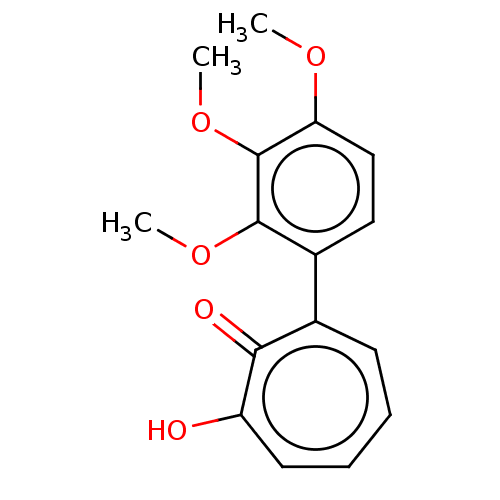
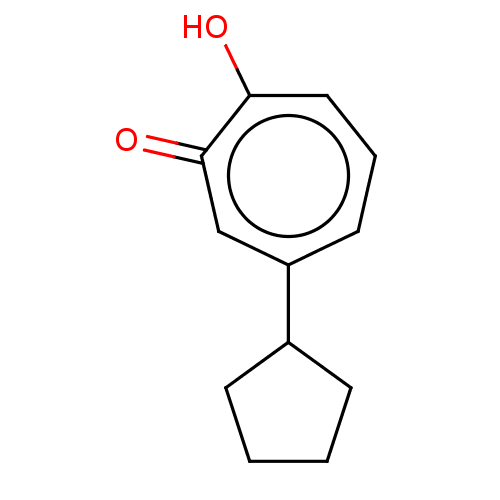
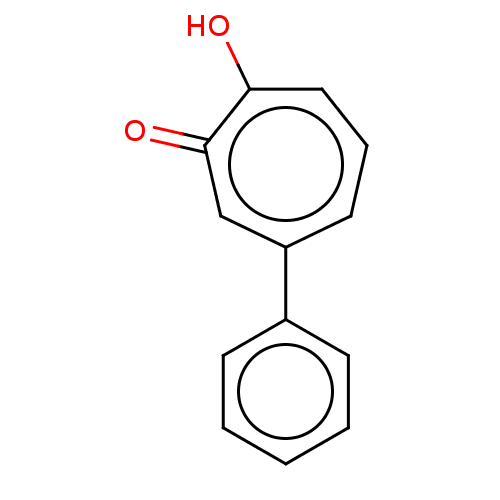
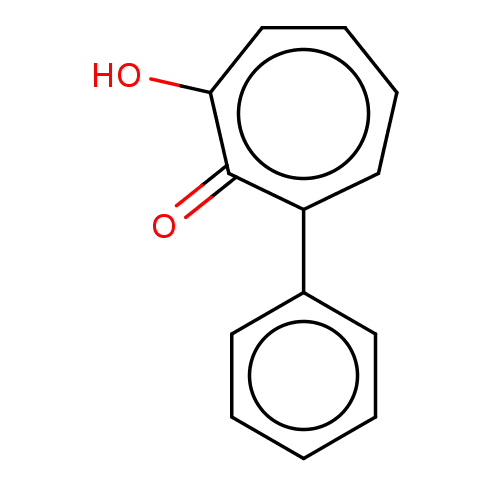
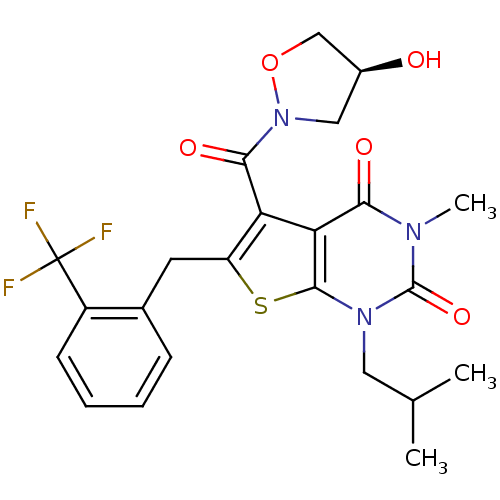
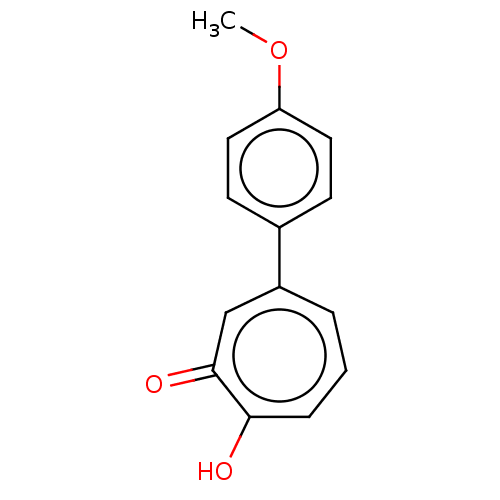
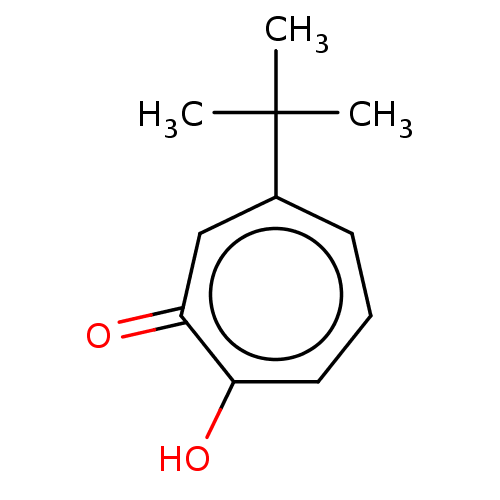
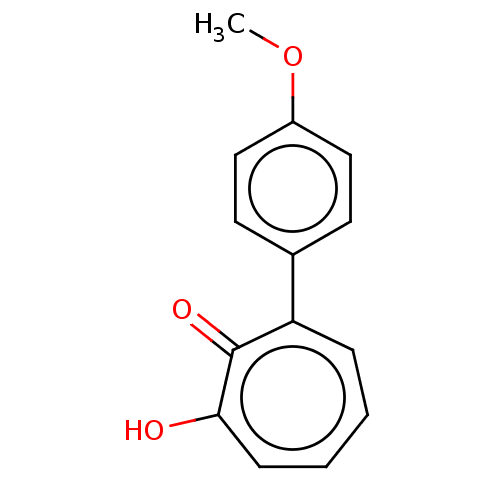
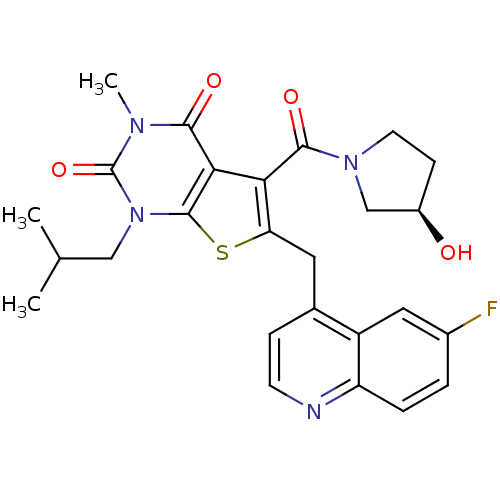
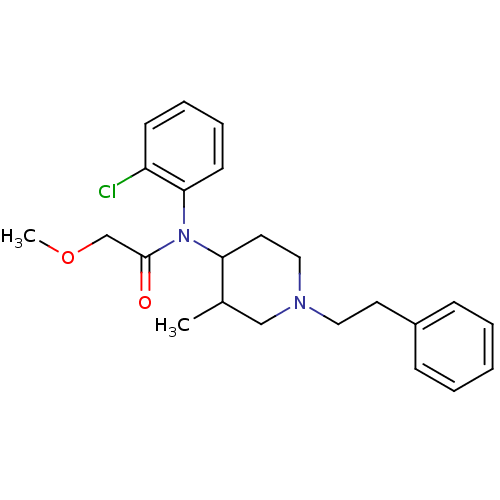
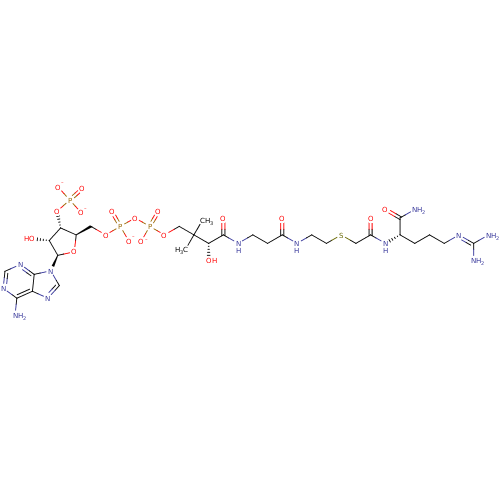
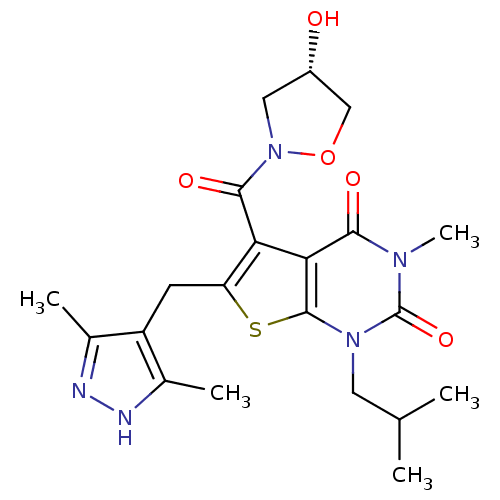
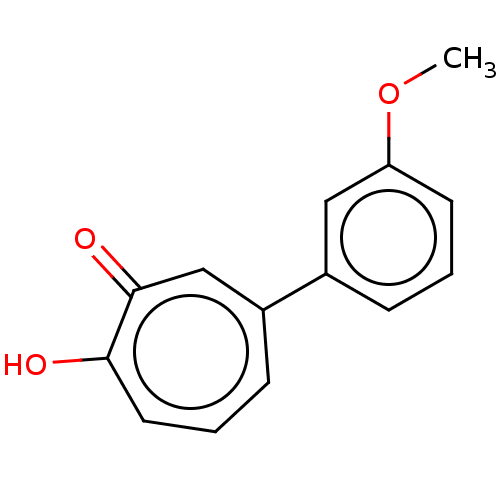
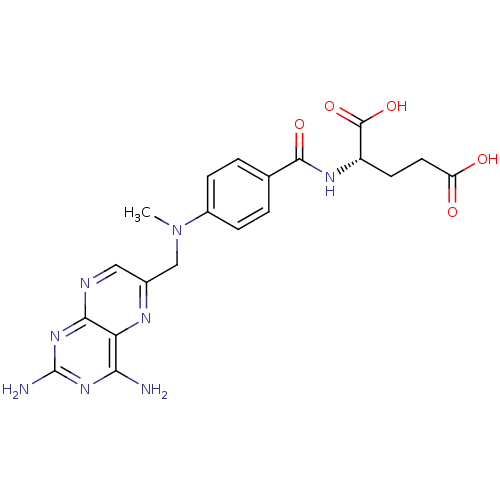
Affinity DataKi: 0.00100nMAssay Description:Inhibition of recombinant Escherichia coli DHFRMore data for this Ligand-Target Pair
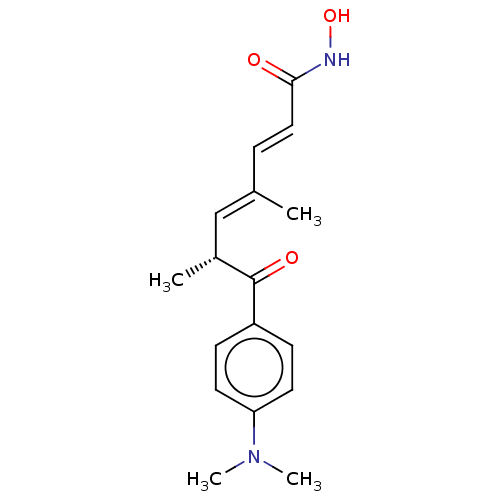
Affinity DataKi: 0.00300nMAssay Description:Inhibition of recombinant human DHFR assessed as reduction in NADPH oxidation using dihydrofolate as substrate by fluorescence spectrophotometric ana...More data for this Ligand-Target Pair
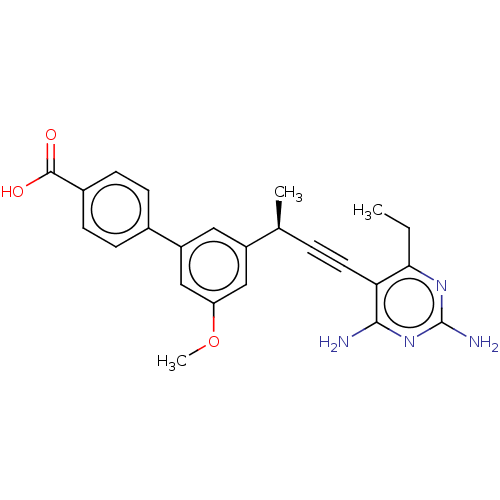
Affinity DataKi: 0.0400nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
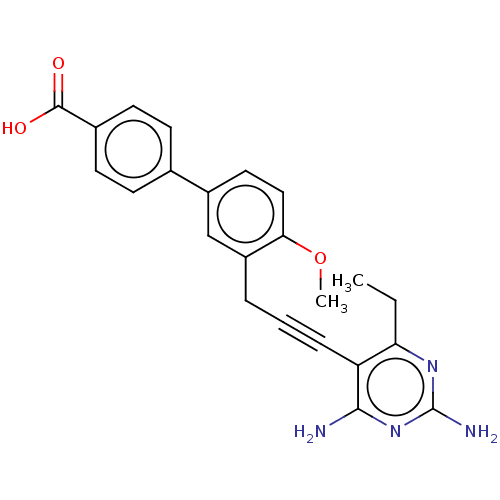
Affinity DataKi: 0.0400nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0600nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.0800nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.0900nM ΔG°: -56.8kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 0.120nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.130nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.140nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.150nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.170nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.190nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.220nMAssay Description:Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof...More data for this Ligand-Target Pair
Affinity DataKi: 0.230nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.25nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.260nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.310nM ΔG°: -53.7kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.420nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.430nM ΔG°: -52.9kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 0.440nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.510nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.520nM ΔG°: -52.5kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 0.530nMAssay Description:Competitive inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.540nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 0.780nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.810nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.810nM ΔG°: -51.4kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 0.870nMAssay Description:Inhibition of human recombinant HDAC1 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 0.910nMAssay Description:Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof...More data for this Ligand-Target Pair
Affinity DataKi: 0.980nMAssay Description:Inhibition of recombinant Escherichia coli DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dihydrof...More data for this Ligand-Target Pair
Affinity DataKi: 1nMAssay Description:Inhibition of Staphylococcus aureus DHFRMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair
Affinity DataKi: 1.10nMAssay Description:Ability to displace [3H]-naloxone from the Opioid receptor mu 1 isolated from the rat brain membranes.More data for this Ligand-Target Pair
Affinity DataKi: 1.20nMAssay Description:Inhibition of Enterococcus faecium AAC(6')IiMore data for this Ligand-Target Pair
Affinity DataKi: 1.20nM ΔG°: -50.4kJ/molepH: 7.8 T: 2°CAssay Description:Jurkat cell membranes were incubated with [3H]-labeled ligand in the absence or presence of increasing concentrations of test compound. The reagents ...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of TMP/methicillin-resistant Staphylococcus aureus wild type DHFR assessed as oxidation of NADPH pre-incubated for 5 mins followed by dihy...More data for this Ligand-Target Pair
Affinity DataKi: 1.30nMAssay Description:Inhibition of recombinant Staphylococcus aureus DHFR expressed in Escherichia coli BL21(DE3) cells assessed as reduction in NADPH oxidation using dih...More data for this Ligand-Target Pair
Affinity DataKi: 1.36nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 1.42nMAssay Description:Key enzyme kinetic parameters for one class I HDAC (HDAC8) and one class Ha HDAC (HDAC4) were determined using commercially available human recombina...More data for this Ligand-Target Pair
Affinity DataKi: 1.5nMAssay Description:Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysisMore data for this Ligand-Target Pair

 3D Structure (crystal)
3D Structure (crystal)