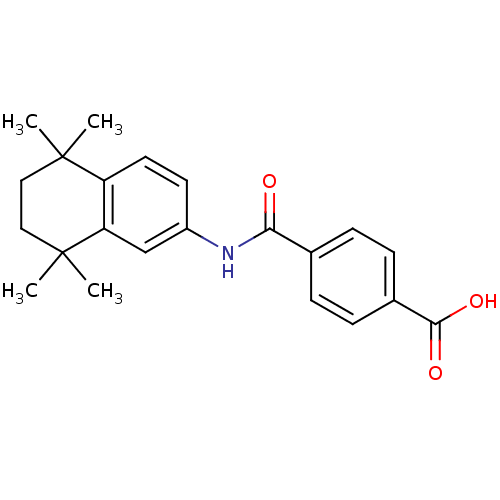
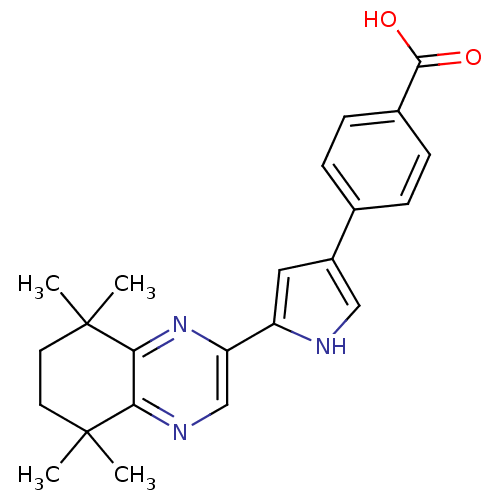
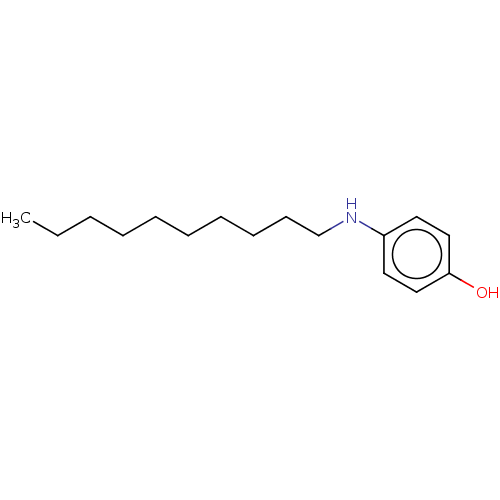
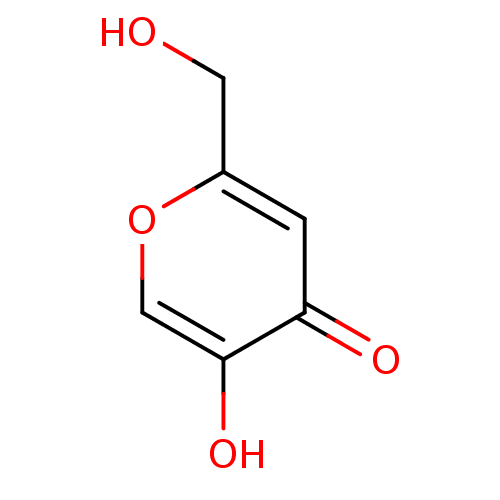
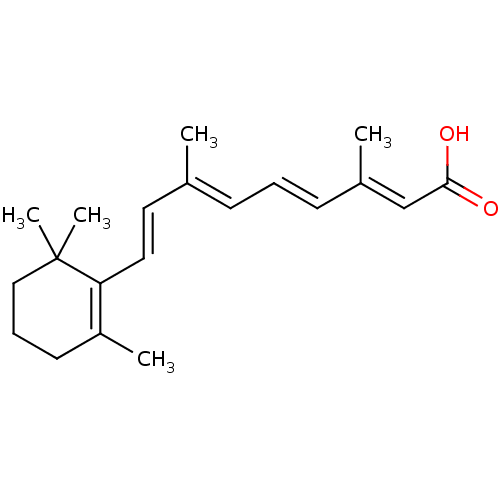
Affinity DataIC50: 0.450nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.620nMAssay Description:Binding affinity against Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.890nMAssay Description:Binding affinity for Retinoic acid receptor alphaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.900nMAssay Description:Inhibition of [3H]-ATRA-Hl60 binding to Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 0.940nMAssay Description:Binding affinity for Retinoic acid receptor betaMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 2.20nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 2.40nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 4.60nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 17nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Binding affinity for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor alpha transactivation by ATRA (50 nM)More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha).More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 78nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 83nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:Binding affinity for Retinoic Acid Receptor alpha (RAR alpha)More data for this Ligand-Target Pair
Affinity DataIC50: 140nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Binding affinity for Retinoic Acid Receptor gamma (RAR gamma)More data for this Ligand-Target Pair
Affinity DataIC50: 230nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 290nMAssay Description:Binding affinity for Retinoic Acid Receptor beta (RAR beta)More data for this Ligand-Target Pair
Affinity DataIC50: 310nMAssay Description:Binding affinity for Retinoic acid receptor gammaMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase kinase 2(Homo sapiens (Human))
Riken Systems and Structural Biology Center
Riken Systems and Structural Biology Center
Affinity DataIC50: 500nMpH: 7.5 T: 2°CAssay Description:The AMPK peptide including the sequence surrounding the phosphorylation site of AMPK (167GEFLRTSCGSP177), was synthesized at the Support Unit for Bio...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+3nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor beta transactivation by ATRA (50 nM); Not detect...More data for this Ligand-Target Pair
Affinity DataIC50: >3.00E+3nMAssay Description:Antagonistic activity of the compound was evaluated in terms of inhibition of Retinoic acid receptor gamma transactivation by ATRA (50 nM); Not detec...More data for this Ligand-Target Pair
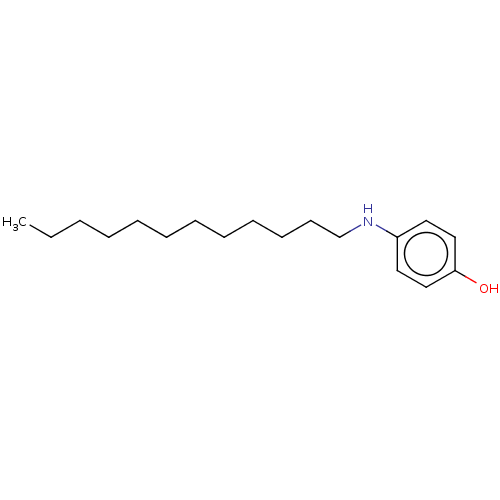
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrate by spectrophotometryMore data for this Ligand-Target Pair
TargetCalcium/calmodulin-dependent protein kinase kinase 2 [ P274E](Homo sapiens (Human))
Riken Systems and Structural Biology Center
Riken Systems and Structural Biology Center
Affinity DataIC50: 2.42E+4nMpH: 7.5 T: 2°CAssay Description:The AMPK peptide including the sequence surrounding the phosphorylation site of AMPK (167GEFLRTSCGSP177), was synthesized at the Support Unit for Bio...More data for this Ligand-Target Pair
Affinity DataIC50: 3.01E+4nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate by spectrophotometry relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: 3.28E+4nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate by spectrophotometry relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of mushroom tyrosinase using L-DOPA as substrate by spectrophotometry relative to controlMore data for this Ligand-Target Pair
Affinity DataIC50: >2.00E+5nMAssay Description:Inhibition of mushroom tyrosinase using L-tyrosine as substrate by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of alpha-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+5nMAssay Description:Inhibition of beta-glucosidaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair

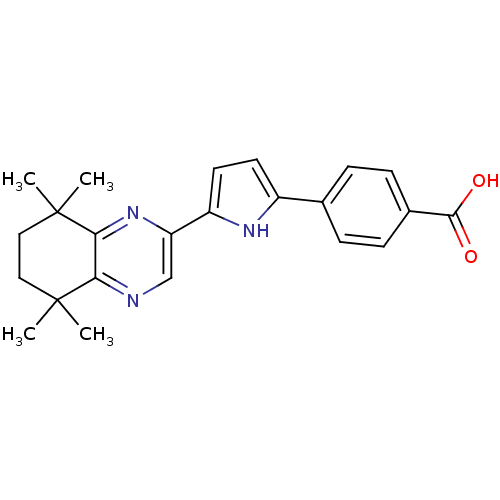

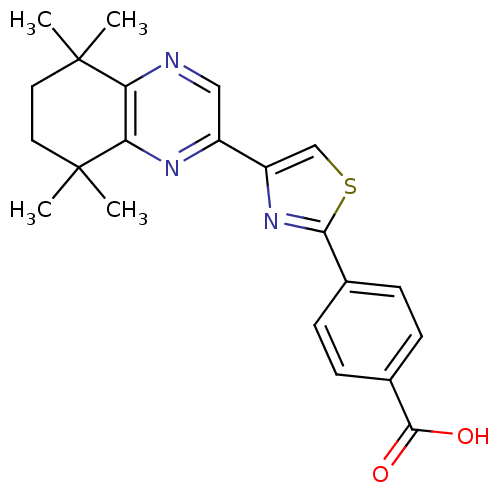
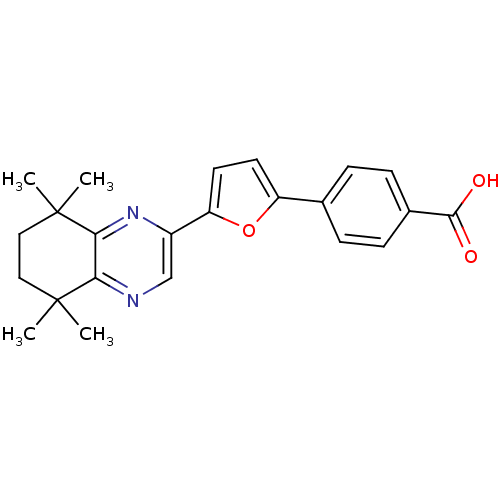
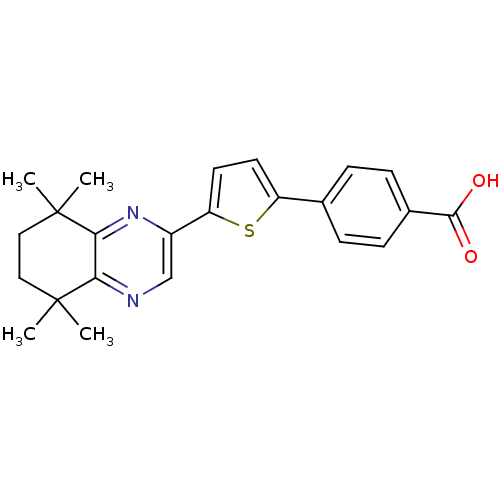

 3D Structure (crystal)
3D Structure (crystal)