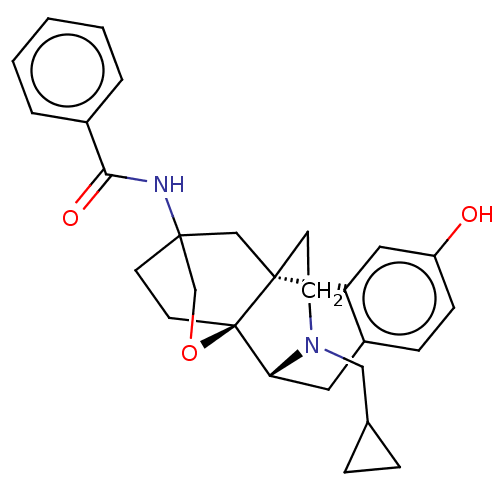
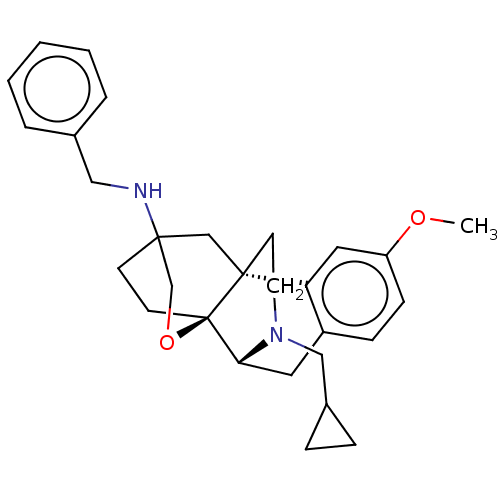
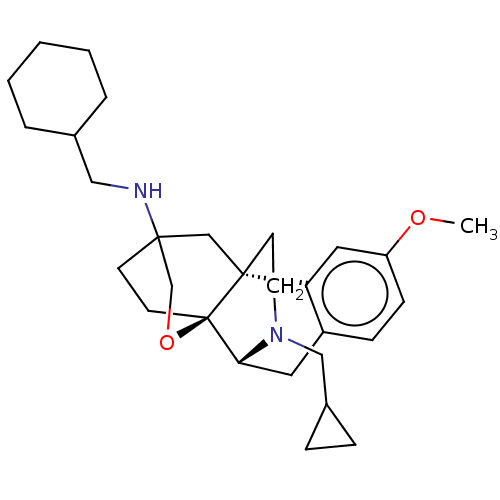
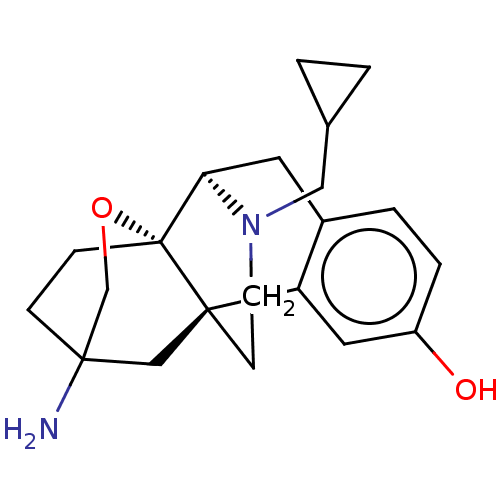
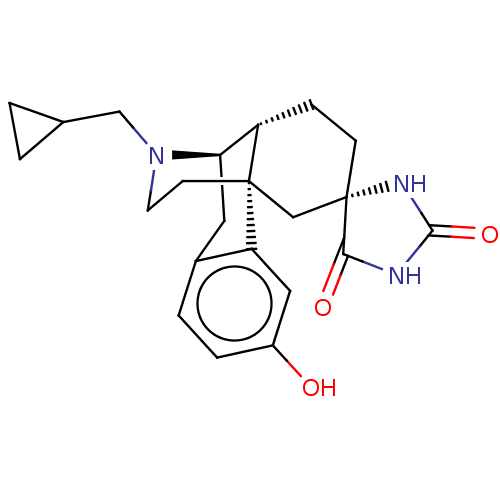
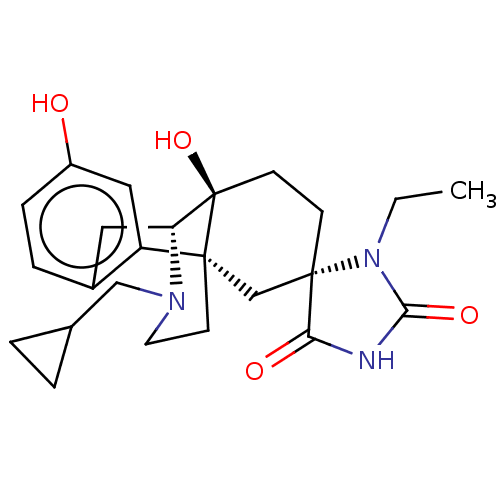
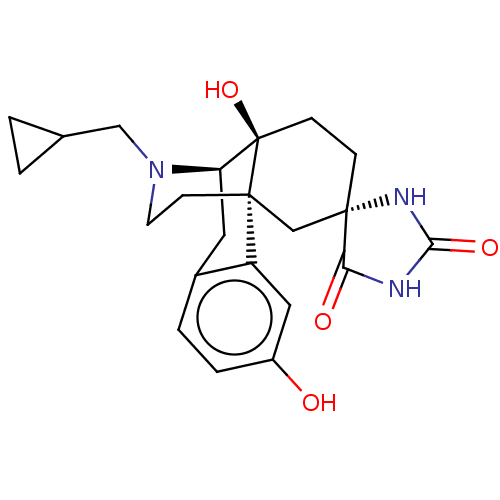
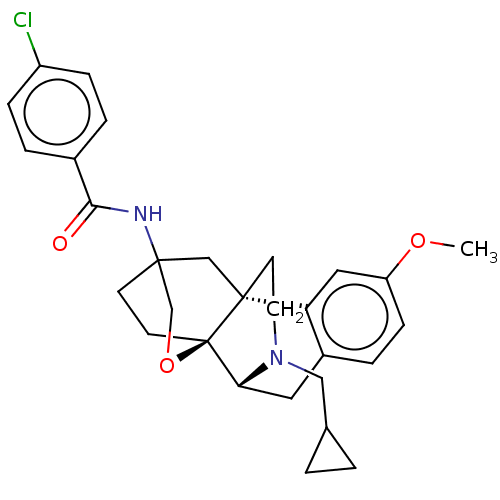
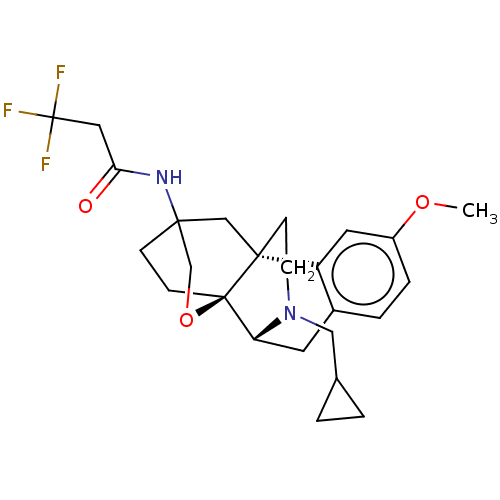
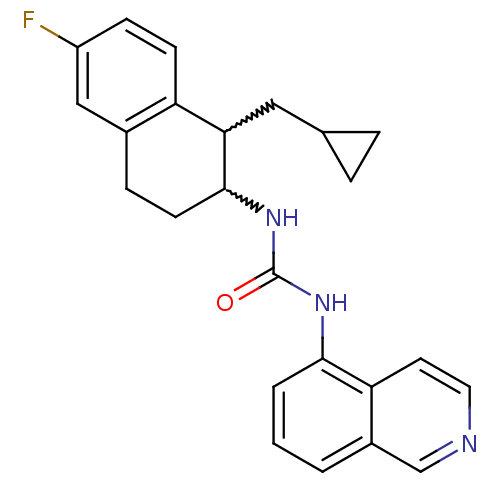
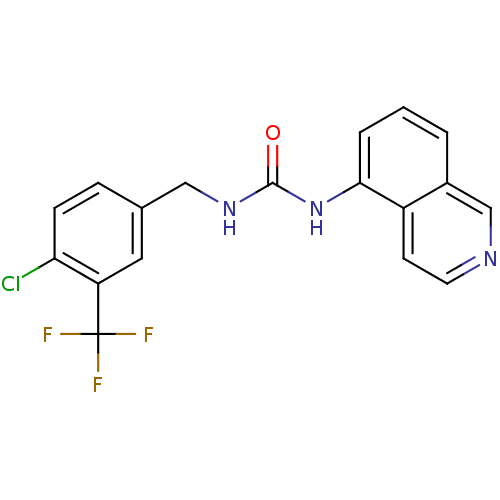
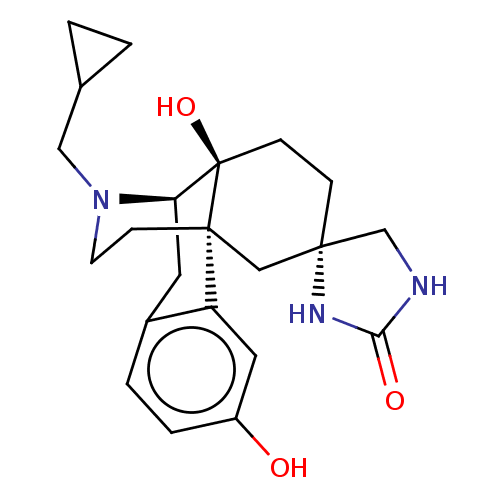
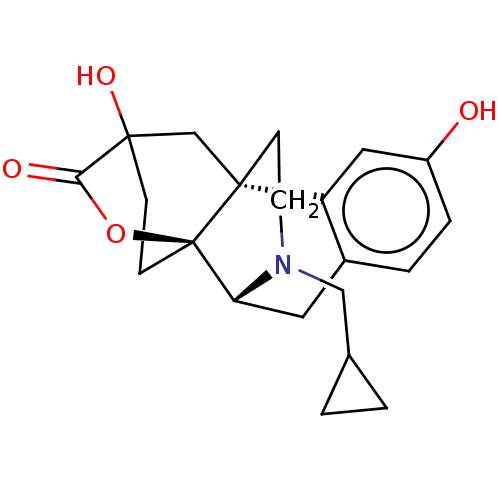
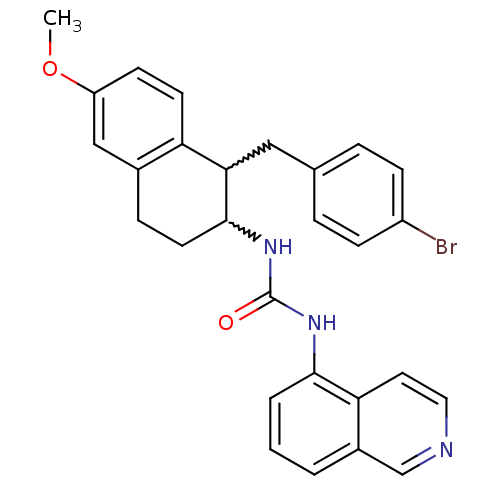
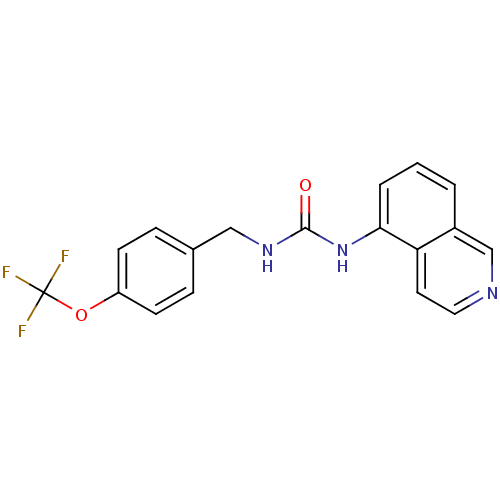
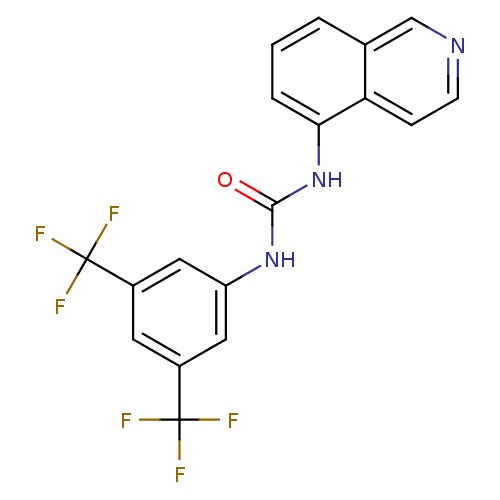
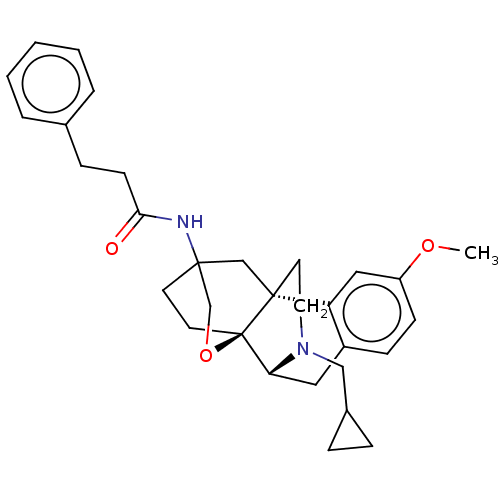
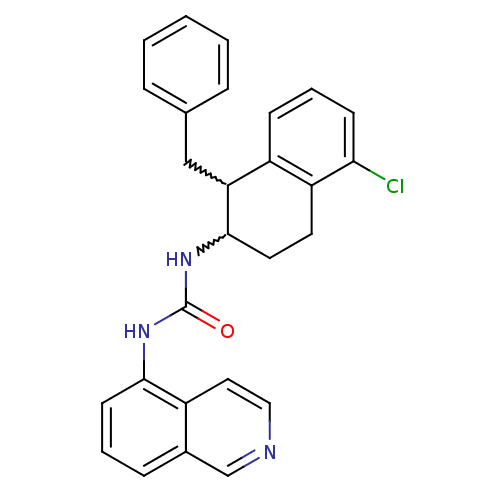
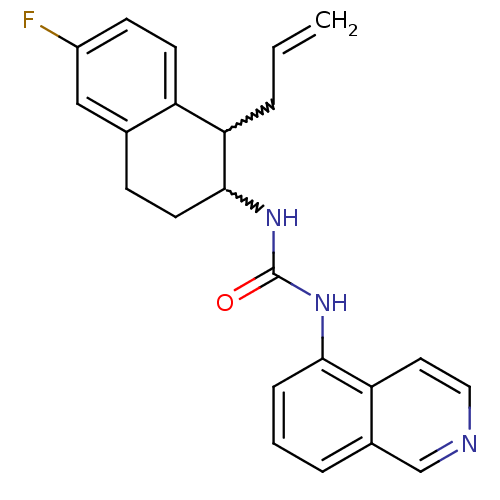
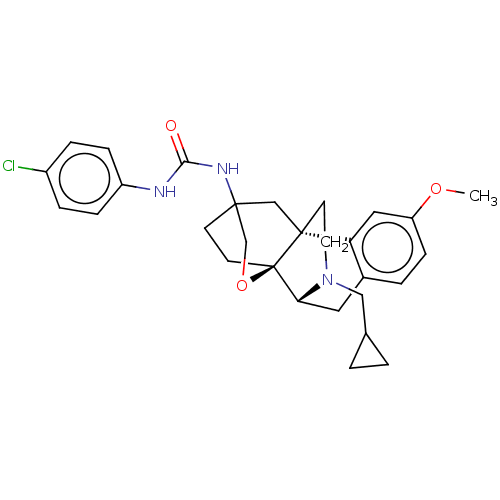
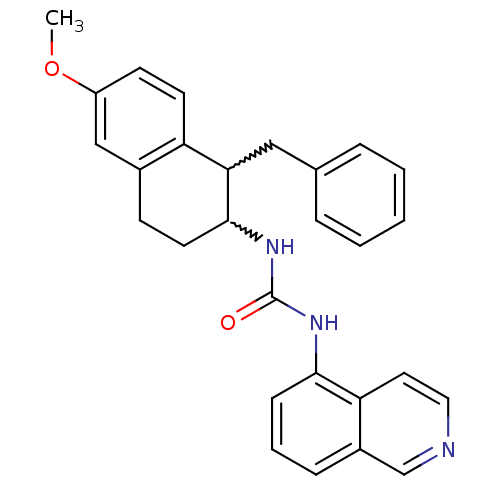
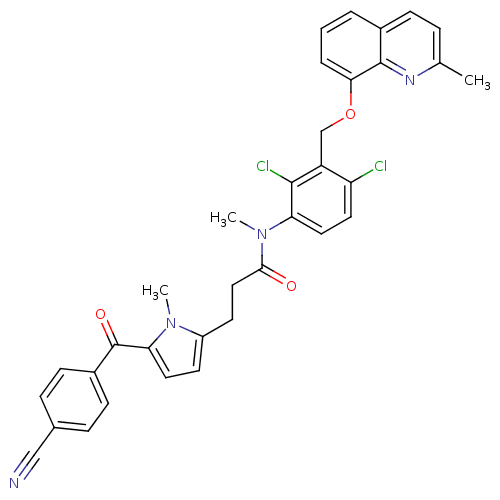
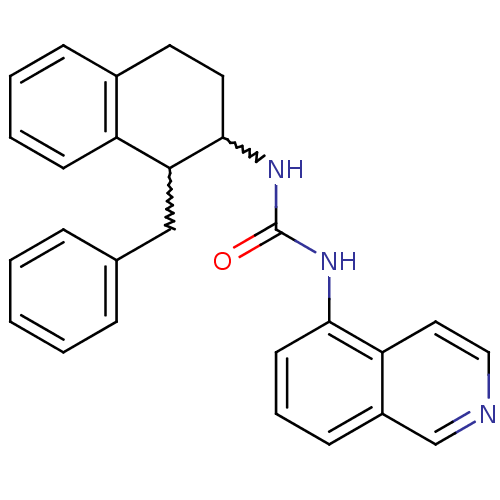
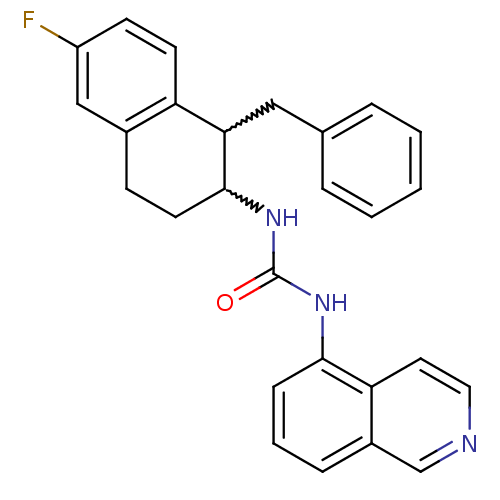
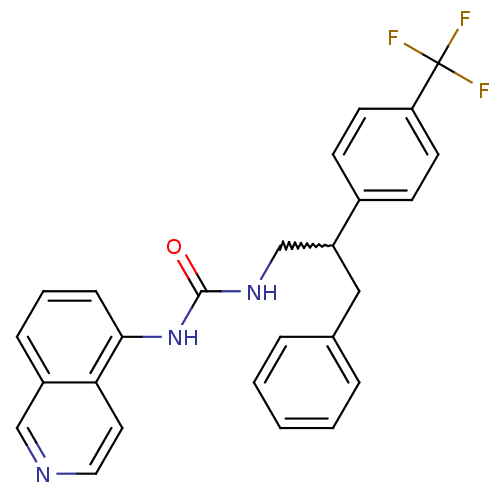
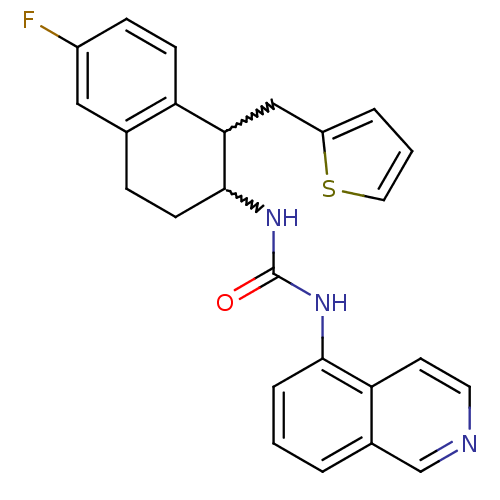
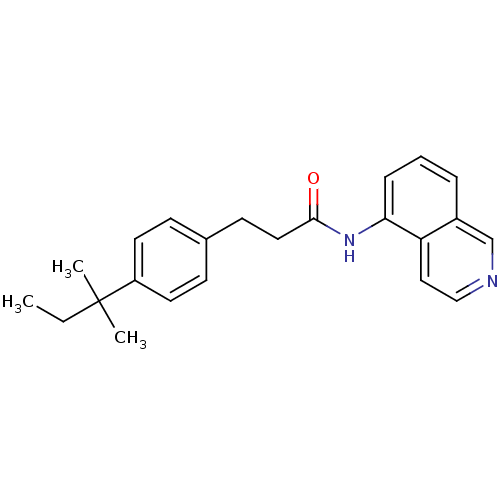
Affinity DataKi: 0.0190nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
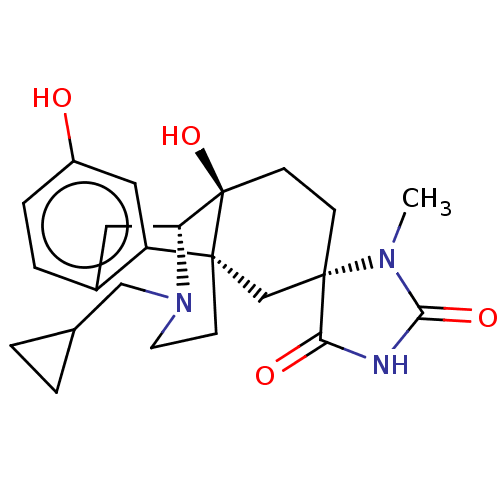
Affinity DataKi: 0.0260nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.0320nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.0520nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
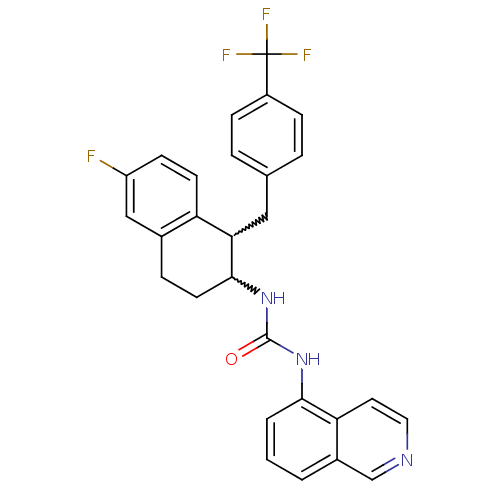
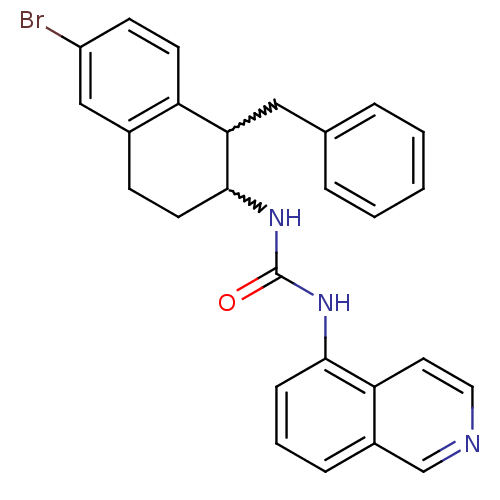
Affinity DataKi: 0.0560nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
Affinity DataKi: 0.0570nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.0590nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.0620nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.0960nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.200nM ΔG°: -55.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.230nM ΔG°: -55.0kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
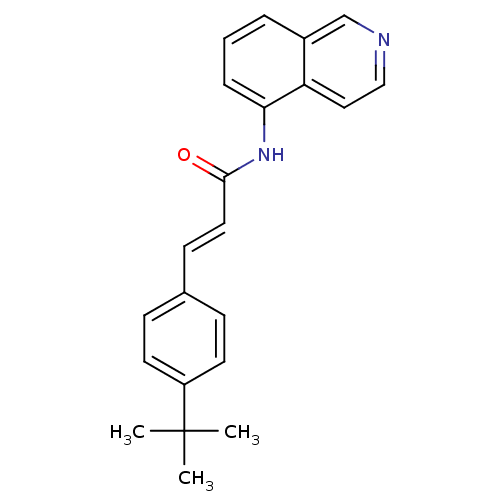
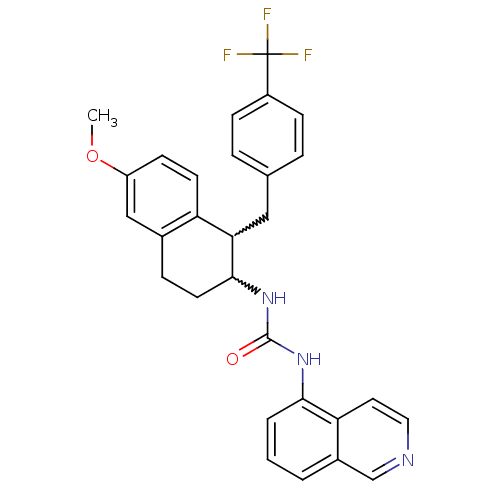
Affinity DataKi: 0.290nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
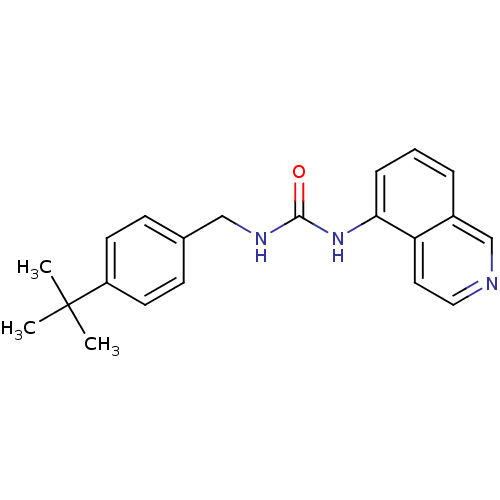
Affinity DataKi: 0.300nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.300nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.300nM ΔG°: -54.4kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.330nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.470nM ΔG°: -53.2kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nM ΔG°: -52.6kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
Affinity DataKi: 0.660nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
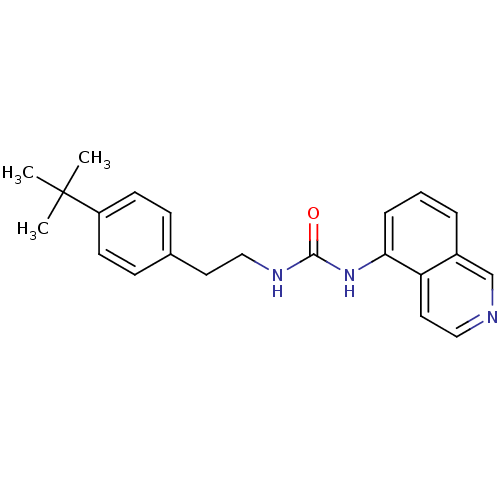
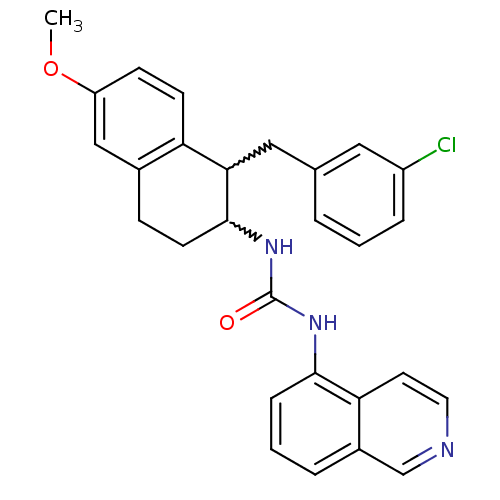
Affinity DataKi: 0.790nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 0.800nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.860nM ΔG°: -56.1kJ/molepH: 7.4 T: 2°CAssay Description:mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per...More data for this Ligand-Target Pair
Affinity DataKi: 0.930nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
Affinity DataKi: 0.940nM ΔG°: -51.5kJ/molepH: 7.4 T: 2°CAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 ug membrane protein (recombinant...More data for this Ligand-Target Pair
Affinity DataKi: 0.950nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.20nMAssay Description:Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.30nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 1.40nMAssay Description:Antagonistic activity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane, as inhibition of agonist-induced intracellular [C...More data for this Ligand-Target Pair
Affinity DataKi: 1.58nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 2nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 2.28nM ΔG°: -53.5kJ/molepH: 7.4 T: 2°CAssay Description:mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per...More data for this Ligand-Target Pair
Affinity DataKi: 2.56nMAssay Description:Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recomb...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 3nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 3.51nMAssay Description:Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg mem...More data for this Ligand-Target Pair
Affinity DataKi: 3.55nM ΔG°: -52.3kJ/molepH: 7.4 T: 2°CAssay Description:mu-Opioid Receptor Binding Assay Procedures: Radioligand dose-displacement binding assays for mu-opioid receptors used 0.3 nM [3H]-diprenorphine (Per...More data for this Ligand-Target Pair
TargetB2 bradykinin receptor(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
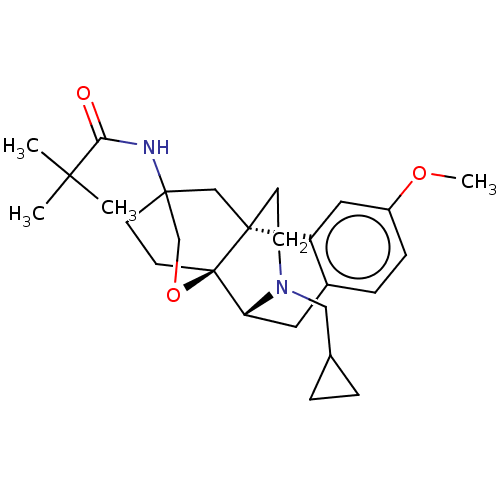
Affinity DataKi: 4nMAssay Description:Binding affinity towards human bradykinin receptor B2More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Displacement of [3H]RTX from human TRPV1 expressed in HEK293 cellsMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 1(Homo sapiens (Human))
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research And Development
Curated by ChEMBL
Affinity DataKi: 4nMAssay Description:Binding affinity towards human vanilloid receptor subtype 1 expressed in HEK293 cell membrane using [3H]-RTX as radioligand.More data for this Ligand-Target Pair