 CAS_58-82-2 NSC_105044 Bradykinin BDBM82076
CAS_58-82-2 NSC_105044 Bradykinin BDBM82076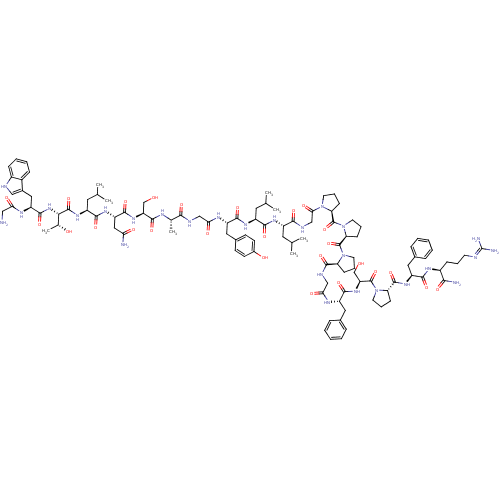 Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35
Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35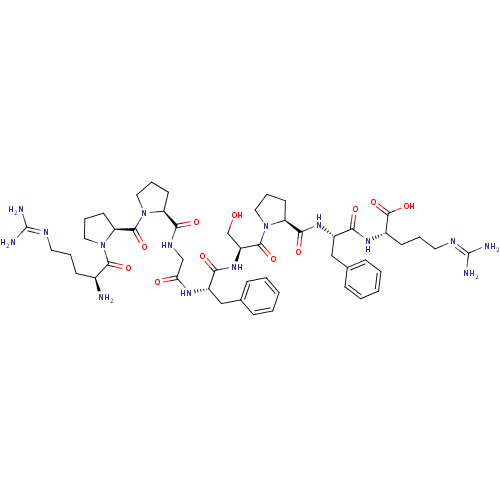 (bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
(bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
- Dankwardt, SM; Ferla, S; Krstenansky, JL; Bhakta, S; Ostrelich, H; Jarnagin, K Nonpeptide bradykinin antagonist analogs based on a model of a Sterling-Winthrop nonpeptide bradykinin antagonist overlapped with cyclic hexapeptide bradykinin antagonist peptides Bioorg Med Chem Lett 7: 1921-1926 (1997)
- Qiu, Y; Taichi, M; Wei, N; Yang, H; Luo, KQ; Tam, JP An Orally Active Bradykinin B J Med Chem 60: 504-510 (2017)
- Barth, M; Bondoux, M; Luccarini, JM; Peyrou, V; Dodey, P; Pruneau, D; Massardier, C; Paquet, JL From bradykinin B2 receptor antagonists to orally active and selective bradykinin B1 receptor antagonists. J Med Chem 55: 2574-84 (2012)
- Kuduk, SD; Ng, C; Feng, DM; Wai, JM; Chang, RS; Harrell, CM; Murphy, KL; Ransom, RW; Reiss, D; Ivarsson, M; Mason, G; Boyce, S; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG 2,3-diaminopyridine bradykinin B1 receptor antagonists. J Med Chem 47: 6439-42 (2004)
- Bryan, MC; Biswas, K; Peterkin, TA; Rzasa, RM; Arik, L; Lehto, SG; Sun, H; Hsieh, FY; Xu, C; Fremeau, RT; Allen, JR Chromenones as potent bradykinin B1 antagonists. Bioorg Med Chem Lett 22: 619-22 (2011)
- Kuduk, SD; Di Marco, CN; Chang, RK; Wood, MR; Kim, JJ; Schirripa, KM; Murphy, KL; Ransom, RW; Tang, C; Torrent, M; Ha, S; Prueksaritanont, T; Pettibone, DJ; Bock, MG 5-Piperazinyl pyridine carboxamide bradykinin B1 antagonists. Bioorg Med Chem Lett 16: 2791-5 (2006)
- Gibson, C; Schnatbaum, K; Pfeifer, JR; Locardi, E; Paschke, M; Reimer, U; Richter, U; Scharn, D; Faussner, A; Tradler, T Novel small molecule bradykinin B2 receptor antagonists. J Med Chem 52: 4370-9 (2009)
- Wood, MR; Kim, JJ; Han, W; Dorsey, BD; Homnick, CF; DiPardo, RM; Kuduk, SD; MacNeil, T; Murphy, KL; Lis, EV; Ransom, RW; Stump, GL; Lynch, JJ; O'Malley, SS; Miller, PJ; Chen, TB; Harrell, CM; Chang, RS; Sandhu, P; Ellis, JD; Bondiskey, PJ; Pettibone, DJ; Freidinger, RM; Bock, MG Benzodiazepines as potent and selective bradykinin B1 antagonists. J Med Chem 46: 1803-6 (2003)
- Bodmer-Narkevitch, V; Anthony, NJ; Cofre, V; Jolly, SM; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG; Kuduk, SD Indazole derivatives as novel bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 20: 7011-4 (2010)
- Cole, AG; Metzger, A; Brescia, MR; Qin, LY; Appell, KC; Brain, CT; Hallett, A; Ganju, P; Denholm, AA; Wareing, JR; Ritchie, TJ; Drake, GM; Bevan, SJ; MacGloinn, A; McBryde, A; Patel, V; Oakley, PJ; Nunez, X; Gstach, H; Schneider, P; Baldwin, JJ; Dolle, RE; McDonald, E; Henderson, I Sulfonamido-aryl ethers as bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 19: 119-22 (2008)
- Douty, BD; Salvino, JM; Seoane, PR; Dolle, RE Synthesis of non-peptide bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 363-366 (1995)
- Dziadulewicz, EK; Ritchie, TJ; Hallett, A; Snell, CR; Davies, JW; Wrigglesworth, R; Dunstan, AR; Bloomfield, GC; Drake, GS; McIntyre, P; Brown, MC; Burgess, GM; Lee, W; Davis, C; Yaqoob, M; Phagoo, SB; Phillips, E; Perkins, MN; Campbell, EA; Davis, AJ; Rang, HP Nonpeptide bradykinin B2 receptor antagonists: conversion of rodent-selective bradyzide analogues into potent, orally-active human bradykinin B2 receptor antagonists. J Med Chem 45: 2160-72 (2002)
- Lee, J; Reynolds, C; Jetter, MC; Youngman, MA; Hlasta, DJ; Dax, SL; Stone, DJ; Zhang, SP; Codd, EE Design and synthesis of novel pyrrolidine-containing bradykinin antagonists. Bioorg Med Chem Lett 13: 1879-82 (2003)
- Su, DS; Lim, JL; Markowitz, MK; Wan, BL; Murphy, KL; Reiss, DR; Harrell, CM; O'Malley, SS; Ransom, RW; Chang, RS; Pettibone, DJ; Tang, C; Prueksaritanont, T; Freidinger, RM; Bock, MG Potent bradykinin B1 receptor antagonists: 4-substituted phenyl cyclohexanes. Bioorg Med Chem Lett 17: 3006-9 (2007)
- Eles, J; Beke, G; Vágó, I; Bozó, E; Huszár, J; Tarcsay, A; Kolok, S; Schmidt, E; Vastag, M; Hornok, K; Farkas, S; Domány, G; Keseru, GM Quinolinyl- and phenantridinyl-acetamides as bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 22: 3095-9 (2012)
- Maggiora, LL; Orawski, AT; Simmons, WH Apstatin analogue inhibitors of aminopeptidase P, a bradykinin-degrading enzyme. J Med Chem 42: 2394-402 (1999)
- Kuduk, SD; Chang, RK; Ng, C; Murphy, KL; Ransom, RW; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG Bradykinin B1 antagonists: SAR studies in the 2,3-diaminopyridine series. Bioorg Med Chem Lett 15: 3925-9 (2005)
- Kuduk, SD; DiPardo, RM; Chang, RK; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Bradykinin B1 antagonists: biphenyl SAR studies in the cyclopropanecarboxamide series. Bioorg Med Chem Lett 17: 3608-12 (2007)
- Huang, H; Player, MR Bradykinin B1 receptor antagonists as potential therapeutic agents for pain. J Med Chem 53: 5383-99 (2010)
- BAEURLE, S; DAVENPORT, A; STIMSON, C; NAGEL, J; SCHMIDT, N; ROTGERI, A; GROETICKE, I; RAUSCH, A; KLAR, J; DYRKS, T CARBOXYLIC ACID AROMATIC AMIDES AS ANTAGONISTS OF BRADYKININ B1 RECEPTOR US Patent US20230271931 (2023)
- Thurieau, C; Félétou, M; Hennig, P; Raimbaud, E; Canet, E; Fauchère, JL Design and synthesis of new linear and cyclic bradykinin antagonists. J Med Chem 39: 2095-101 (1996)
- Biswas, K; Peterkin, TA; Bryan, MC; Arik, L; Lehto, SG; Sun, H; Hsieh, FY; Xu, C; Fremeau, RT; Allen, JR Discovery of potent, orally bioavailable phthalazinone bradykinin B1 receptor antagonists. J Med Chem 54: 7232-46 (2011)
- Salvino, JM; Seoane, PR; Douty, BD; Awad, MA; Hoyer, D; Ross, TM; Dolle, RE; Houck, WT; Faunce, DM; Sawutz, DG Structure activity relationships of non-peptide bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 357-362 (1995)
- Su, DS; Lim, JL; Tinney, E; Wan, BL; Murphy, KL; Reiss, DR; Harrell, CM; O'Malley, SS; Ransom, RW; Chang, RS; Pettibone, DJ; Yu, J; Tang, C; Prueksaritanont, T; Freidinger, RM; Bock, MG; Anthony, NJ 2-Aminobenzophenones as a novel class of bradykinin B1 receptor antagonists. J Med Chem 51: 3946-52 (2008)
- Amblard, M; Daffix, I; Bedos, P; Bergé, G; Pruneau, D; Paquet, JL; Luccarini, JM; Bélichard, P; Dodey, P; Martinez, J Design and synthesis of potent bradykinin agonists containing a benzothiazepine moiety. J Med Chem 42: 4185-92 (1999)
- Schnatbaum, K; Schaudt, M; Stragies, R; Pfeifer, JR; Gibson, C; Locardi, E; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Zischinsky, G Novel small molecule bradykinin B1 receptor antagonists. Part 3: hydroxyurea derivatives. Bioorg Med Chem Lett 20: 1233-6 (2010)
- Youngman, MA; Carson, JR; Lee, JS; Dax, SL; Zhang, SP; Colburn, RW; Stone, DJ; Codd, EE; Jetter, MC Synthesis and structure--activity relationships of aroylpyrrole alkylamide bradykinin (B2) antagonists. Bioorg Med Chem Lett 13: 1341-4 (2003)
- Wood, MR; Schirripa, KM; Kim, JJ; Kuduk, SD; Chang, RK; Di Marco, CN; DiPardo, RM; Wan, BL; Murphy, KL; Ransom, RW; Chang, RS; Holahan, MA; Cook, JJ; Lemaire, W; Mosser, SD; Bednar, RA; Tang, C; Prueksaritanont, T; Wallace, AA; Mei, Q; Yu, J; Bohn, DL; Clayton, FC; Adarayn, ED; Sitko, GR; Leonard, YM; Freidinger, RM; Pettibone, DJ; Bock, MG Alpha-hydroxy amides as a novel class of bradykinin B1 selective antagonists. Bioorg Med Chem Lett 18: 716-20 (2008)
- Schaudt, M; Locardi, E; Zischinsky, G; Stragies, R; Pfeifer, JR; Gibson, C; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Schnatbaum, K Novel small molecule bradykinin B1 receptor antagonists. Part 1: benzamides and semicarbazides. Bioorg Med Chem Lett 20: 1225-8 (2010)
- Zischinsky, G; Stragies, R; Schaudt, M; Pfeifer, JR; Gibson, C; Locardi, E; Scharn, D; Richter, U; Kalkhof, H; Dinkel, K; Schnatbaum, K Novel small molecule bradykinin B1 receptor antagonists. Part 2: 5-membered diaminoheterocycles. Bioorg Med Chem Lett 20: 1229-32 (2010)
- Stahl, W; Breipohl, G; Kuhlmann, L; Steinsträsser, A; Gerhards, HJ; Schölkens, BA Technetium-99m-labeled HOE 140: a potential bradykinin B2 receptor imaging agent. J Med Chem 38: 2799-801 (1995)
- Hoyer, D; Awad, MM; Salvino, JM; Seoane, PR; Dolle, RE; Houck, WT; Sawutz, DG Ace inhibitors as a template for the design of bradykinin B2 receptor antagonists Bioorg Med Chem Lett 5: 367-370 (1995)
- Biswas, K; Aya, T; Qian, W; Peterkin, TA; Chen, JJ; Human, J; Hungate, RW; Kumar, G; Arik, L; Lester-Zeiner, D; Biddlecome, G; Manning, BH; Sun, H; Dong, H; Huang, M; Loeloff, R; Johnson, EJ; Askew, BC Aryl sulfones as novel bradykinin B1 receptor antagonists for treatment of chronic pain. Bioorg Med Chem Lett 18: 4764-9 (2008)
- Kuduk, SD; Chang, RK; Dipardo, RM; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Bradykinin B1 receptor antagonists: an alpha-hydroxy amide with an improved metabolism profile. Bioorg Med Chem Lett 18: 5107-10 (2008)
- Salvino, JM; Seoane, PR; Douty, BD; Awad, MM; Dolle, RE; Houck, WT; Faunce, DM; Sawutz, DG Design of potent non-peptide competitive antagonists of the human bradykinin B2 receptor. J Med Chem 36: 2583-4 (1993)
- Chakravarty, S; Wilkins, D; Kyle, DJ Design of potent, cyclic peptide bradykinin receptor antagonists from conformationally constrained linear peptides. J Med Chem 36: 2569-71 (1993)
- Su, DS; Markowitz, MK; Murphy, KL; Wan, BL; Zrada, MM; Harrell, CM; O'Malley, SS; Hess, JF; Ransom, RW; Chang, RS; Wallace, MA; Raab, CE; Dean, DC; Pettibone, DJ; Freidinger, RM; Bock, MG Development of an efficient and selective radioligand for bradykinin B1 receptor occupancy studies. Bioorg Med Chem Lett 14: 6045-8 (2004)
- Kuduk, SD; Di Marco, CN; Chang, RK; Wood, MR; Schirripa, KM; Kim, JJ; Wai, JM; DiPardo, RM; Murphy, KL; Ransom, RW; Harrell, CM; Reiss, DR; Holahan, MA; Cook, J; Hess, JF; Sain, N; Urban, MO; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG Development of orally bioavailable and CNS penetrant biphenylaminocyclopropane carboxamide bradykinin B1 receptor antagonists. J Med Chem 50: 272-82 (2007)
- Chen, JJ; Qian, W; Biswas, K; Viswanadhan, VN; Askew, BC; Hitchcock, S; Hungate, RW; Arik, L; Johnson, E Discovery of dihydroquinoxalinone acetamides containing bicyclic amines as potent Bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 18: 4477-81 (2008)
- Amblard, M; Daffix, I; Bergé, G; Calmès, M; Dodey, P; Pruneau, D; Paquet, JL; Luccarini, JM; Bélichard, P; Martinez, J Synthesis and characterization of bradykinin B(2) receptor agonists containing constrained dipeptide mimics. J Med Chem 42: 4193-201 (1999)
- Guo, Q; Chandrasekhar, J; Ihle, D; Wustrow, DJ; Chenard, BL; Krause, JE; Hutchison, A; Alderman, D; Cheng, C; Cortright, D; Broom, D; Kershaw, MT; Simmermacher-Mayer, J; Peng, Y; Hodgetts, KJ 1-Benzylbenzimidazoles: the discovery of a novel series of bradykinin B(1) receptor antagonists. Bioorg Med Chem Lett 18: 5027-31 (2008)
- Feng, DM; Wai, JM; Kuduk, SD; Ng, C; Murphy, KL; Ransom, RW; Reiss, D; Chang, RS; Harrell, CM; MacNeil, T; Tang, C; Prueksaritanont, T; Freidinger, RM; Pettibone, DJ; Bock, MG 2,3-Diaminopyridine as a platform for designing structurally unique nonpeptide bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 15: 2385-8 (2005)
- Liu, Q; Qian, W; Li, A; Biswas, K; Chen, JJ; Fotsch, C; Han, N; Yuan, C; Arik, L; Biddlecome, G; Johnson, E; Kumar, G; Lester-Zeiner, D; Ng, GY; Hungate, RW; Askew, BC Aryl sulfonamides containing tetralin allylic amines as potent and selective bradykinin B1 receptor antagonists. Bioorg Med Chem Lett 20: 4593-7 (2010)
- Deekonda, S; Rankin, D; Davis, P; Lai, J; Porreca, F; Hruby, VJ Design, synthesis and biological evaluation of multifunctional ligands targeting opioid and bradykinin 2 receptors. Bioorg Med Chem Lett 25: 4148-52 (2015)
- Huszár, J; Timár, Z; Szalai, KK; Keseru, G; Fülöp, F; Penke, B Novel bradykinin-1 antagonists containing a (1,2,3,4-tetrahydro-isoquinolin-1-yl)acetic acid scaffold. Eur J Med Chem 43: 1552-8 (2008)
- Fotsch, C; Biddlecome, G; Biswas, K; Chen, JJ; D'Amico, DC; Groneberg, RD; Han, NB; Hsieh, FY; Kamassah, A; Kumar, G; Lester-Zeiner, D; Liu, Q; Mareska, DA; Riahi, BB; Wang, YJ; Yang, K; Zhan, J; Zhu, J; Johnson, E; Ng, G; Askew, BC A new class of bradykinin 1 receptor antagonists containing the piperidine acetic acid tetralin core. Bioorg Med Chem Lett 16: 2071-5 (2006)
- D'Amico, DC; Aya, T; Human, J; Fotsch, C; Chen, JJ; Biswas, K; Riahi, B; Norman, MH; Willoughby, CA; Hungate, R; Reider, PJ; Biddlecome, G; Lester-Zeiner, D; Staden, CV; Johnson, E; Kamassah, A; Arik, L; Wang, J; Viswanadhan, VN; Groneberg, RD; Zhan, J; Suzuki, H; Toro, A; Mareska, DA; Clarke, DE; Harvey, DM; Burgess, LE; Laird, ER; Askew, B; Ng, G Identification of a nonpeptidic and conformationally restricted bradykinin B1 receptor antagonist with anti-inflammatory activity. J Med Chem 50: 607-10 (2007)
- Kyle, DJ; Blake, PR; Smithwick, D; Green, LM; Martin, JA; Sinsko, JA; Summers, MF NMR and computational evidence that high-affinity bradykinin receptor antagonists adopt C-terminal beta-turns. J Med Chem 36: 1450-60 (1993)
- Dressen, D; Garofalo, AW; Hawkinson, J; Hom, D; Jagodzinski, J; Marugg, JL; Neitzel, ML; Pleiss, MA; Szoke, B; Tung, JS; Wone, DW; Wu, J; Zhang, H Preparation and optimization of a series of 3-carboxamido-5-phenacylaminopyrazole bradykinin B1 receptor antagonists. J Med Chem 50: 5161-7 (2007)
- Artis, DR; Brotherton-Pleiss, C; Pease, JH; Lin, CJ; Ferla, SW; Newman, SR; Bhakta, S; Ostrelich, H; Jarnagin, K Structure-based design of six novel classes of nonpeptide antagonists of the bradykinin B2 receptor. Bioorg Med Chem Lett 10: 2421-5 (2001)
- Dziadulewicz, EK; Brown, MC; Dunstan, AR; Lee, W; Said, NB; Garratt, PJ The design of non-peptide human bradykinin B2 receptor antagonists employing the benzodiazepine peptidomimetic scaffold. Bioorg Med Chem Lett 9: 463-8 (1999)
- Feng, DM; DiPardo, RM; Wai, JM; Chang, RK; Di Marco, CN; Murphy, KL; Ransom, RW; Reiss, DR; Tang, C; Prueksaritanont, T; Pettibone, DJ; Bock, MG; Kuduk, SD A new class of bradykinin B1 receptor antagonists with high oral bioavailability and minimal PXR activity. Bioorg Med Chem Lett 18: 682-7 (2008)
- Bedos, P; Amblard, M; Subra, G; Dodey, P; Luccarini, JM; Paquet, JL; Pruneau, D; Aumelas, A; Martinez, J A rational approach to the design and synthesis of a new bradykinin B(1) receptor antagonist. J Med Chem 43: 2387-94 (2000)
- Qian, W; Chen, JJ; Human, J; Aya, T; Zhu, J; Biswas, K; Peterkin, T; Hungate, RW; Arik, L; Johnson, E; Kumar, G; Joseph, S; Jona, J; Guo, HX; Wu, Z Discovery of dehydro-oxopiperazine acetamides as novel bradykinin B1 receptor antagonists with enhanced in vitro potency. Bioorg Med Chem Lett 22: 1061-7 (2012)
- Biswas, K; Li, A; Chen, JJ; D'Amico, DC; Fotsch, C; Han, N; Human, J; Liu, Q; Norman, MH; Riahi, B; Yuan, C; Suzuki, H; Mareska, DA; Zhan, J; Clarke, DE; Toro, A; Groneberg, RD; Burgess, LE; Lester-Zeiner, D; Biddlecome, G; Manning, BH; Arik, L; Dong, H; Huang, M; Kamassah, A; Loeloff, R; Sun, H; Hsieh, FY; Kumar, G; Ng, GY; Hungate, RW; Askew, BC; Johnson, E Potent nonpeptide antagonists of the bradykinin B1 receptor: structure-activity relationship studies with novel diaminochroman carboxamides. J Med Chem 50: 2200-12 (2007)
- Amblard, M; Bedos, P; Olivier, C; Daffix, I; Luccarini, JM; Dodey, P; Pruneau, D; Paquet, JL; Martinez, J Synthesis and biological evaluation of bradykinin B(1)/B(2) and selective B(1) receptor antagonists. J Med Chem 43: 2382-6 (2000)
- Kyle, DJ; Chakravarty, S; Sinsko, JA; Stormann, TM A proposed model of bradykinin bound to the rat B2 receptor and its utility for drug design. J Med Chem 37: 1347-54 (1994)
- Guo, XM; Yadav, MB; Khan, M; Hao, CW; Lin, CY; Huang, T; Wu, J; Fan, BM; Bian, ZX Bradykinin-Potentiating Peptide-Paclitaxel Conjugate Directed at Ectopically Expressed Angiotensin-Converting Enzyme in Triple-Negative Breast Cancer. J Med Chem 64: 17051-17062 (2021)
- Tancredi, M; Galoppini, C; Meini, S; Quartara, L; Maggi, CA; Rovero, P Synthesis and biological activity of new bradykinin pseudopeptide B1 receptor agonists containing alkylic spacers Bioorg Med Chem Lett 7: 2661-2664 (1997)
- Chen, JJ; Nguyen, T; D'Amico, DC; Qian, W; Human, J; Aya, T; Biswas, K; Fotsch, C; Han, N; Liu, Q; Nishimura, N; Peterkin, TA; Yang, K; Zhu, J; Riahi, BB; Hungate, RW; Andersen, NG; Colyer, JT; Faul, MM; Kamassah, A; Wang, J; Jona, J; Kumar, G; Johnson, E; Askew, BC 3-Oxo-2-piperazinyl acetamides as potent bradykinin B1 receptor antagonists for the treatment of pain and inflammation. Bioorg Med Chem Lett 21: 3384-9 (2011)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Imai, K; Inamura, N; Asano, M; Hatori, C; Katayama, A; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 1. Construction of the basic framework. J Med Chem 41: 564-78 (1998)
- Heitsch, H; Wagner, A; Schölkens, BA; Wirth, K Novel series of O-substituted 8-quinolines and 4-benzothiazoles as potent antagonists of the bradykinin B2 receptors. Bioorg Med Chem Lett 9: 327-32 (1999)
- Rassias, G; Leonardi, S; Rigopoulou, D; Vachlioti, E; Afratis, K; Piperigkou, Z; Koutsakis, C; Karamanos, NK; Gavras, H; Papaioannou, D Potent antiproliferative activity of bradykinin B2 receptor selective agonist FR-190997 and analogue structures thereof: A paradox resolved? Eur J Med Chem 210: (2021)
- Fincham, CI; Bressan, A; D'Andrea, P; Ettorre, A; Giuliani, S; Mauro, S; Meini, S; Paris, M; Quartara, L; Rossi, C; Squarcia, A; Valenti, C; Daniela, F; Maggi, CA Design and synthesis of novel sulfonamide-containing bradykinin hB(2) receptor antagonists. Synthesis and structure-relationships ofa,a-tetrahydropyranylglycine. Bioorg Med Chem 20: 2091-100 (2012)
- Mavunkel, BJ; Lu, Z; Goehring, RR; Lu, S; Chakravarty, S; Perumattam, J; Novotny, EA; Connolly, M; Valentine, H; Kyle, DJ Synthesis and characterization of pseudopeptide bradykinin B2 receptor antagonists containing the 1,3,8-triazaspiro[4.5]decan-4-one ring system. J Med Chem 39: 3169-73 (1996)
- Dziadulewicz, EK; Ritchie, TJ; Hallett, A; Snell, CR; Ko, SY; Wrigglesworth, R; Hughes, GA; Dunstan, AR; Bloomfield, GC; Drake, GS; Brown, MC; Lee, W; Burgess, GM; Davis, C; Yaqoob, M; Perkins, MN; Campbell, EA; Davis, AJ; Rang, HP 1-(2-Nitrophenyl)thiosemicarbazides: a novel class of potent, orally active non-peptide antagonist for the bradykinin B(2) receptor. J Med Chem 43: 769-71 (2000)
- Wood, MR; Schirripa, KM; Kim, JJ; Wan, BL; Murphy, KL; Ransom, RW; Chang, RS; Tang, C; Prueksaritanont, T; Detwiler, TJ; Hettrick, LA; Landis, ER; Leonard, YM; Krueger, JA; Lewis, SD; Pettibone, DJ; Freidinger, RM; Bock, MG Cyclopropylamino acid amide as a pharmacophoric replacement for 2,3-diaminopyridine. Application to the design of novel bradykinin B1 receptor antagonists. J Med Chem 49: 1231-4 (2006)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Aramori, I; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 4. Discovery of novel frameworks mimicking the active conformation. J Med Chem 41: 4587-98 (1998)
- Goodfellow, VS; Marathe, MV; Kuhlman, KG; Fitzpatrick, TD; Cuadrado, D; Hanson, W; Zuzack, JS; Ross, SE; Wieczorek, M; Burkard, M; Whalley, ET Bradykinin receptor antagonists containing N-substituted amino acids: in vitro and in vivo B(2) and B(1) receptor antagonist activity. J Med Chem 39: 1472-84 (1996)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 2. Overcoming the species difference between guinea pig and man. J Med Chem 41: 4053-61 (1998)
- Abe, Y; Kayakiri, H; Satoh, S; Inoue, T; Sawada, Y; Inamura, N; Asano, M; Aramori, I; Hatori, C; Sawai, H; Oku, T; Tanaka, H A novel class of orally active non-peptide bradykinin B2 receptor antagonists. 3. Discovering bioisosteres of the imidazo[1,2-a] pyridine moiety. J Med Chem 41: 4062-79 (1998)
- Ritchie, TJ; Dziadulewicz, EK; Culshaw, AJ; Müller, W; Burgess, GM; Bloomfield, GC; Drake, GS; Dunstan, AR; Beattie, D; Hughes, GA; Ganju, P; McIntyre, P; Bevan, SJ; Davis, C; Yaqoob, M Potent and orally bioavailable non-peptide antagonists at the human bradykinin B(1) receptor based on a 2-alkylamino-5-sulfamoylbenzamide core. J Med Chem 47: 4642-4 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Mizutani, T; Inamura, N; Asano, M; Hatori, C; Aramori, I; Oku, T; Tanaka, H Discovery of the first non-peptide full agonists for the human bradykinin B(2) receptor incorporating 4-(2-picolyloxy)quinoline and 1-(2-picolyl)benzimidazole frameworks. J Med Chem 47: 2853-63 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Imai, K; Mizutani, T; Inamura, N; Asano, M; Aramori, I; Hatori, C; Katayama, A; Oku, T; Tanaka, H A new series of highly potent non-peptide bradykinin B2 receptor antagonists incorporating the 4-heteroarylquinoline framework. Improvement of aqueous solubility and new insights into species difference. J Med Chem 47: 1617-30 (2004)
- Sawada, Y; Kayakiri, H; Abe, Y; Mizutani, T; Inamura, N; Asano, M; Aramori, I; Hatori, C; Oku, T; Tanaka, H A new class of nonpeptide bradykinin B(2) receptor ligand, incorporating a 4-aminoquinoline framework. Identification of a key pharmacophore to determine species difference and agonist/antagonist profile. J Med Chem 47: 2667-77 (2004)
- ChEMBL_40276 (CHEMBL653409) Binding affinity towards bradykinin receptor B2 using [3H]bradykinin
- ChEMBL_1559755 (CHEMBL3779535) Displacement of [3H]bradykinin from human recombinant B2 bradykinin receptor
- ChEBML_40423 Binding affinity against human bradykinin receptor B2 using [3H]bradykinin as radioligand
- ChEMBL_40423 (CHEMBL652638) Binding affinity against human bradykinin receptor B2 using [3H]bradykinin as radioligand
- ChEMBL_429986 (CHEMBL917716) Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO cells
- ChEBML_40275 Binding affinity against [3H]bradykinin binding to bradykinin receptor B2 in guinea pig ileal membrane
- ChEMBL_425212 (CHEMBL855158) Displacement of [3H]bradykinin from human bradykinin B2 receptor expressed in CHO-K1 cells
- ChEMBL_643606 (CHEMBL1212470) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells
- ChEMBL_806223 (CHEMBL1958764) Displacement of [3H][desArg9]Lys-Bradykinin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_40426 (CHEMBL652641) Binding affinity towards human cloned Bradykinin receptor B2 expressed in CHO cells by [3H]bradykinin displacement.
- ChEMBL_40441 (CHEMBL652380) Binding affinity against rat Bradykinin receptor B2 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_40442 (CHEMBL652381) Binding affinity against rat bradykinin B2 receptors expressed in CHO cells using [3H]bradykinin as radioligand
- ChEMBL_40130 (CHEMBL658496) Binding affinity against human cloned Bradykinin receptor B1 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_40422 (CHEMBL652637) Binding affinity against human cloned Bradykinin receptor B2 expressed in CHO cells using [3H]-bradykinin as radioligand
- ChEMBL_965476 (CHEMBL2395280) Displacement of [3H]Bradykinin from human recombinant bradykinin B2 receptor expressed in CHEM1 cells after 60 mins
- Binding Assay Binding assay using Bradykinin-1 receptor.
- ChEBML_40424 Binding affinity towards human bradykinin receptor B2
- ChEMBL_333385 (CHEMBL859129) Binding affinity to bradykinin B2 receptor
- ChEMBL_39982 (CHEMBL653556) Inhibition of human bradykinin B1 receptor
- ChEMBL_423710 (CHEMBL913022) Inhibition of human bradykinin B2 receptor
- ChEMBL_535289 (CHEMBL982614) Inhibition of human bradykinin B1 receptor
- ChEMBL_662793 (CHEMBL1252532) Inhibition of rabbit bradykinin B1 receptor
- ChEMBL_662794 (CHEMBL1252533) Inhibition of rat bradykinin B1 receptor
- ChEMBL_879298 (CHEMBL2208696) Antagonist activity at Bradykinin B1 receptor
- ChEMBL_497315 (CHEMBL995877) Displacement of [3H]Lys-bradykinin from human recombinant bradykinin B1 receptor expressed in CHO cells by scintillation counting
- ChEBML_40429 In vitro binding affinity against Bradykinin receptor B2
- ChEMBL_2297250 Binding affinity to B2 bradykinin receptor (unknown origin)
- ChEMBL_302466 (CHEMBL826332) Binding affinity against Human bradykinin receptor B1
- ChEMBL_423681 (CHEMBL853755) Binding affinity to rat bradykinin B1 receptor
- ChEMBL_423682 (CHEMBL853760) Binding affinity to rabbit bradykinin B1 receptor
- ChEMBL_429982 (CHEMBL917712) Binding affinity to rabbit bradykinin B1 receptor
- ChEMBL_434373 (CHEMBL919421) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_434374 (CHEMBL919418) Antagonist activity at rabbit bradykinin B1 receptor
- ChEMBL_443978 (CHEMBL893140) Binding affinity at human bradykinin B1 receptor
- ChEMBL_487377 (CHEMBL1021904) Binding affinity to human bradykinin B1 receptor
- ChEMBL_535281 (CHEMBL982606) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_662796 (CHEMBL1252535) Binding affinity to human bradykinin B1 receptor
- ChEMBL_662797 (CHEMBL1252536) Antagonist activity at dog bradykinin B1 receptor
- ChEMBL_662798 (CHEMBL1252537) Binding affinity to rat bradykinin B1 receptor
- ChEMBL_662799 (CHEMBL1252538) Binding affinity to mouse bradykinin B1 receptor
- ChEMBL_662800 (CHEMBL1252539) Binding affinity to monkey bradykinin B1 receptor
- ChEMBL_748611 (CHEMBL1780464) Antagonist activity at human bradykinin B1 receptor
- ChEMBL_796433 (CHEMBL1937748) Binding affinity to human B1 bradykinin receptor
- ChEMBL_814368 (CHEMBL2019577) Binding affinity to human bradykinin B1 receptor
- ChEMBL_814371 (CHEMBL2019580) Antagonist activity at rat bradykinin B1 receptor
- ChEMBL_610511 (CHEMBL1064982) Antagonist activity at bradykinin B1 receptor in human IMR90 cells assessed as inhibition of des-Arg-bradykinin-mediated calcium mobilization
- ChEMBL_612385 (CHEMBL1066271) Antagonist activity at bradykinin B1 receptor in human IMR90 cells assessed as inhibition of des-Arg-bradykinin-mediated calcium mobilization
- ChEMBL_806221 (CHEMBL1958762) Displacement of [3H]-Bradykinin from human bradykinin B2 receptor expressed in CHO cells membrane after 60 mins by scintillation counting
- ChEMBL_806231 (CHEMBL1958772) Antagonist activity at guinea pig bradykinin B2 receptor in longitudinal smooth muscle assessed as inhibition of bradykinin-induced contractile responses
- ChEMBL_523549 (CHEMBL1001563) Antagonist activity at human recombinant bradykinin 2 receptor expressed in CHO cells assessed as inhibition of bradykinin-induced intracellular calcium mobilization
- Calcium Mobilization Assay Calcium mobilization assay using Bradykinin-1 receptor.
- ChEMBL_2078239 (CHEMBL4734030) Agonist activity at bradykinin B2 receptor (unknown origin)
- ChEMBL_423683 (CHEMBL853765) Binding affinity to rhesus monkey bradykinin B1 receptor
- ChEMBL_658534 (CHEMBL1247736) Binding affinity to human recombinant bradykinin B1 receptor
- ChEMBL_658535 (CHEMBL1247737) Binding affinity to human recombinant bradykinin B2 receptor
- ChEMBL_1633556 (CHEMBL3876348) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells measured after 60 mins by scintillation counting method
- ChEMBL_1861205 (CHEMBL4362061) Displacement of [3H]bradykinin from human recombinant bradykinin B2 receptor expressed in CHO cells incubated for 2 hrs by liquid scintillation counter
- ChEMBL_806222 (CHEMBL1958763) Antagonist activity at human bradykinin B2 receptor expressed in dhfr-deficient CHO cells assessed as inhibition of bradykinin-induced inositol monophosphate accumulation preincubated for 15 mins prior bradykinin induction measured after 40 mins by liquid scintillation spectrometry
- ChEMBL_305717 (CHEMBL829390) Inhibition of Bradykinin receptor B1 expressed in HEK293 cells
- ChEMBL_572632 (CHEMBL1026056) Displacement of [3H]bradykinin from wild-type B2 receptor
- ChEMBL_572638 (CHEMBL1026062) Displacement of [3H]bradykinin from guinea pig B2 receptor
- ChEBML_40431 Tested for binding affinity against human IMR-90 Bradykinin receptor B2
- ChEMBL_2023082 (CHEMBL4676895) Displacement of [3H]nicotine from B2 bradykinin receptor (unknown origin)
- ChEMBL_302814 (CHEMBL839504) Binding affinity for Bradykinin receptor B2 expressed in COS7 cells
- ChEMBL_302842 (CHEMBL827887) Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells
- ChEMBL_321573 (CHEMBL881788) Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived
- ChEMBL_40138 (CHEMBL876936) Compound was tested for inhibition against rat Bradykinin receptor B1
- ChEMBL_429983 (CHEMBL917713) Activity at rabbit bradykinin B1 receptor assessed by FLIPR assay
- ChEMBL_443979 (CHEMBL893141) Antagonist activity in human bradykinin B1 receptor by FLIPR method
- ChEMBL_463210 (CHEMBL947207) Binding affinity to human bradykinin B1 receptor by FLIPR assay
- ChEBML_40277 In vitro binding affinity against bradykinin receptor B2 from guinea pig ileum.
- ChEBML_40427 Binding affinity against human IMR 90 fetal lung fibroblast bradykinin receptor B2
- ChEMBL_1561510 (CHEMBL3776147) Inhibition of recombinant human bradykinin receptor 2 expressed in CHO cells
- ChEMBL_321543 (CHEMBL881481) Inhibitory concentration against endothelin converting enzyme 1 using bradykinin-derived substrate
- ChEMBL_333384 (CHEMBL859128) Binding affinity to human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_40431 (CHEMBL652645) Tested for binding affinity against human IMR-90 Bradykinin receptor B2
- ChEMBL_463209 (CHEMBL947206) Binding affinity to human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463211 (CHEMBL947208) Binding affinity to rat bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463212 (CHEMBL947209) Binding affinity to monkey bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463213 (CHEMBL947210) Binding affinity to rabbit bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463214 (CHEMBL947211) Binding affinity to dog bradykinin B1 receptor expressed in CHO cells
- ChEMBL_463823 (CHEMBL948902) Binding affinity to human bradykinin B1 receptor expressed in rat CNS
- ChEMBL_644658 (CHEMBL1211637) Displacement of [3H]Lys-desArg9-BK from human bradykinin B1 receptor
- ChEMBL_796432 (CHEMBL1937747) Antagonist activity at rabbit B1 bradykinin receptor expressed in CHO cells
- ChEMBL_964329 (CHEMBL2395591) Binding affinity to human bradykinin B2 receptor by radioligand displacement assay
- ChEMBL_966197 (CHEMBL2395702) Binding affinity to human bradykinin B2 receptor by radioligand displacement assay
- ChEBML_40106 Binding affinity against human IMR90 fetal lung fibroblast bradykinin B2 receptor was evaluated
- ChEBML_40141 Compound was evaluated for competitive [3H]bradykinin binding to guinea pig ileum homogenates
- ChEMBL_302733 (CHEMBL838681) Binding affinity of the [35S]- radiolabeled compound to dog Bradykinin receptor B1
- ChEMBL_302734 (CHEMBL876354) Binding affinity of the [35S]- radiolabeled compound to rat Bradykinin receptor B1
- ChEMBL_302791 (CHEMBL839475) Binding affinity of the [35S]- radiolabeled compound to rabbit Bradykinin receptor B1
- ChEMBL_40139 (CHEMBL656133) Antagonistic activity against Bradykinin receptor B1 of rat ileum longitudinal smooth muscle.
- ChEMBL_40427 (CHEMBL652642) Binding affinity against human IMR 90 fetal lung fibroblast bradykinin receptor B2
- ChEMBL_40437 (CHEMBL652376) Inhibition of Bradykinin receptor B2-mediated contractions of rat uterus smooth muscle
- ChEMBL_302897 (CHEMBL830212) Binding affinity of the [35S]- radiolabeled compound to rhesus monkey Bradykinin receptor B1
- ChEMBL_302988 (CHEMBL830227) Inhibitory constant against human Bradykinin receptor B1 expressed in chinese hamster ovary cells
- ChEMBL_39981 (CHEMBL653555) Compound was tested for inhibition against human bradykinin B1 receptor using FLIPR assay
- ChEMBL_40135 (CHEMBL656787) In vitro Bradykinin receptor B1 antagonist activity in functional tissue within rabbit aorta
- ChEMBL_40137 (CHEMBL656789) Compound was tested for inhibition against rat Bradykinin receptor B1 using FLIPR assay
- ChEMBL_40279 (CHEMBL653412) Antagonistic activity against Bradykinin receptor B2 of guinea pig ileum longitudinal smooth muscle.
- ChEMBL_40283 (CHEMBL653415) Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (WI38 fibroblasts)
- ChEMBL_40445 (CHEMBL652384) In vitro Bradykinin receptor B2 antagonist activity by using rat uterus functional assay
- ChEMBL_434360 (CHEMBL919419) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHOD cells
- ChEMBL_510232 (CHEMBL1005610) Antagonist activity at human bradykinin B1 receptor expressed in CHO-D-/aequorin cells
- ChEMBL_572637 (CHEMBL1026061) Displacement of [3H]bradykinin from human recombinant B2 receptor expressed in HEK293 cells
- ChEBML_40297 Compound was assayed for binding against the human Bradykinin receptor B2 expressed in CHO cells
- ChEMBL_40271 (CHEMBL656506) In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig pulmonary artery assay.
- ChEMBL_40285 (CHEMBL653417) Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (WI38 fibroblasts)
- ChEMBL_425211 (CHEMBL855157) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D cells
- ChEBML_1711424 Displacement of [3H]bradykinin from human recombinant B2 receptor after 60 mins by scintillation counting analysis
- ChEMBL_40270 (CHEMBL656505) In vitro antagonistic activity for Bradykinin receptor B2 by guinea pig ileal membrane receptor assay.
- ChEMBL_40282 (CHEMBL653414) Antagonism of the [Ca2+] efflux actions of human Bradykinin receptor B2 (SK-N-SH neuroblastoma)
- ChEMBL_40428 (CHEMBL652643) Ability to bind to human cloned B2 receptor in competition binding experiments with [3H]- bradykinin
- ChEMBL_425214 (CHEMBL855160) Antagonistic activity at rat bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_429981 (CHEMBL917711) Displacement of [3H]des-arg10 kallidin from rat bradykinin B1 receptor expressed in CHO cells
- ChEMBL_446485 (CHEMBL895597) Displacement of [3H]DAKA from human bradykinin B1 receptor in IL1-beta stimulated IMR90 cells
- ChEMBL_1861204 (CHEMBL4362060) Displacement of [3H]BK from Bradykinin B2 Receptor in guinea pig ileum by liquid scintillation counter
- ChEMBL_216334 (CHEMBL818748) Inhibition of specific binding of [3H]BK to bradykinin B2 receptors of guinea pig ileum (GPI)
- ChEMBL_216335 (CHEMBL818749) Inhibition of specific binding of [3H]BK to bradykinin B2 receptors of guinea pig ileum (GPI)
- ChEMBL_306532 (CHEMBL827821) Antagonist activity against human Bradykinin receptor B1 was determined in a fluorescence imaging plate reader assay
- ChEMBL_40267 (CHEMBL656503) Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the bradykinin receptor B2
- ChEMBL_40284 (CHEMBL653416) Antagonism of the [Ca2+] efflux actions of the human Bradykinin receptor B2 (SK-N-SH neuroblastoma)
- ChEMBL_40430 (CHEMBL876526) Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells
- ChEMBL_423668 (CHEMBL913433) Displacement of [3H]des-Arg10 Leu9 kallidin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_429973 (CHEMBL917703) Displacement of [3H]des-arg10, leu9-kallidin from human bradykinin B1 receptor expressed in CHO cells
- ChEMBL_446484 (CHEMBL895596) Antagonist activity at human bradykinin B1 receptor in IL1-beta stimulated IMR90 cells by FLIPR assay
- ChEMBL_512956 (CHEMBL977292) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells
- ChEMBL_662792 (CHEMBL1252531) Displacement of [3H]Lys0-des-Arg9-BK at human bradykinin B1 receptor expressed in HEK293 cells
- ChEMBL_40291 (CHEMBL653423) Inhibition of [3H]BK (1.0 nM) binding to the human bradykinin receptor B2, expressed in CHO cells
- ChEMBL_40419 (CHEMBL652634) Compound was assayed for binding against the human Bradykinin receptor B2 expressed in CHO cells; 0.2-16
- ChEMBL_40421 (CHEMBL652636) Affinity to human Bradykinin receptor B2 in CHO cell membranes determined by displacement of [3H]-NPC 17731
- ChEMBL_425235 (CHEMBL855800) Antagonistic activity at African green monkey bradykinin B1 receptor assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_572639 (CHEMBL1026063) Antagonist activity at B2 receptor in human HF15 cells assessed as inhibition of bradykinin-induced calcium mobilization
- ChEMBL_644657 (CHEMBL1211636) Antagonist activity at human bradykinin B1 receptor assessed as inhibition of Lys-desArg9-BK-induced calcium flux
- ChEMBL_662795 (CHEMBL1252534) Antagonist activity at human bradykinin B1 receptor in human MR5 cells assessed as [3H]inositol phosphate accumulation
- ChEMBL_796434 (CHEMBL1937749) Antagonist activity at human B1 bradykinin receptor expressed in CHO cells by aqueorin-based calcium flux assay
- ChEMBL_965477 (CHEMBL2395281) Displacement of [3H](Des-Arg10)-Kallidin from bradykinin B1 receptor in human IMR90 cells after 60 mins
- ChEMBL_40274 (CHEMBL656509) Inhibition of the specific binding of [3H]BK to Bradykinin receptor B2 in guinea pig ileum membrane preparations
- ChEMBL_40294 (CHEMBL884128) Inhibition of the specific binding of [3H]BK to human recombinant Bradykinin receptor B2 expressed in CHO cells.
- ChEMBL_40438 (CHEMBL652377) Inhibition of increase in [Ca2+] efflux from NG108-15 cells caused by activation of rat Bradykinin receptor B2
- ChEMBL_512957 (CHEMBL977293) Antagonist activity at human bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells by aquerin based assay
- ChEMBL_512960 (CHEMBL977296) Antagonist activity at rabbit bradykinin B1 receptor expressed in clone CHO-D-/aequorin cells by aquerin based assay
- ChEMBL_302253 (CHEMBL830269) Equilibrium dissociation constant of the [35S]- radiolabeled compound against human Bradykinin receptor B1 expressed in CHO membranes was determined
- ChEMBL_425210 (CHEMBL855156) Antagonistic activity at human bradykinin B1 receptor expressed in CHO cells assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_434361 (CHEMBL919420) Antagonist activity at human bradykinin B1 receptor expressed in CHOD cells assessed as effect on DAK-induced calcium flux
- ChEMBL_510231 (CHEMBL1005609) Displacement of [3H]DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells by rapid filtration technique
- ChEMBL_535280 (CHEMBL982605) Antagonist activity at Cynomolgus monkey bradykinin B1 receptor expressed in CHO cells assessed as calcium transient by FLIPR assay
- ChEMBL_756380 (CHEMBL1805406) Antagonist activity at human bradykinin B1 receptor expressed in human HEK293 cells assessed as calcium flux by FLIPR assay
- ChEBML_40107 Binding affinity towards human bradykinin B2 receptor (membranes from Cos-7 cells expressing human B2 receptor) using [3H]-BK as radioligand
- ChEMBL_303697 (CHEMBL828184) Binding affinity to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_303698 (CHEMBL828185) Binding affinity to human Bradykinin receptor B2 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_40443 (CHEMBL652382) Inhibition of binding of [3H]BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes
- ChEMBL_425213 (CHEMBL855159) Antagonistic activity at rabbit bradykinin B1 receptor expressed in CHO-D cells assessed as effect on DAK-mediated calcium mobilization
- ChEMBL_492574 (CHEMBL952241) Displacement of [3H]des-arg10leu9kallidin from human bradykinin B1 receptor expressed in CHO cells by Wallac beta-plate scintillation counting
- ChEMBL_1560590 (CHEMBL3777322) Displacement of [3H]BK from cloned human B2 bradykinin receptor expressed in CHO cells after 2 hrs by liquid scintillation counter
- ChEMBL_40269 (CHEMBL856079) Concentration required to inhibit specific binding of [3H]-BK(0.06 nM) to the bradykinin receptor B2 in guinea pig ileum membrane
- ChEMBL_40292 (CHEMBL653424) In vitro inhibitory activity towards human bradykinin receptor B2 expressed in CHO cells using [3H]BK (1.0 nM) as a radioligand
- ChEMBL_40293 (CHEMBL653425) Inhibition of specific binding of [3H]BK at 1 nM to human bradykinin receptor B2 expressed in CHO cells by 50%.
- ChEMBL_40444 (CHEMBL652383) Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes
- ChEMBL_852821 (CHEMBL2155420) Antagonist activity at human bradykinin B1 receptor expressed in human CHO cells assessed as inhibition of calcium flux by FLIPR assay
- ChEBML_40436 In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay
- ChEMBL_40262 (CHEMBL653278) In vitro for inhibition of specific binding of [3H]BK (0.06 nM) to bradykinin receptor B2 in guinea pig ileum membrane preparations.
- ChEMBL_40272 (CHEMBL656507) In vitro inhibitory activity towards bradykinin receptor B2 using [3H]BK (0.06 nM) as a radioligand in guinea pig ileum membrane preparation
- ChEMBL_40273 (CHEMBL656508) Inhibition of specific binding of [3H]BK at 0.06 nM to bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_612393 (CHEMBL1066279) Antagonist activity at bradykinin B1 receptor in human IMR90 cells pretreated with IL1-beta assessed as inhibition of DAKD-induced calcium mobilization
- ChEMBL_797691 (CHEMBL1943911) Displacement of [3H]-DAK from human bradykinin B1 receptor expressed in CHO-D-/aequorin cells membrane after 90 mins by scintillation counting
- ChEMBL_1550090 (CHEMBL3756036) Inhibition of Pseudomonas aeruginosa pseudolysin pretreated with compound for 1 hr followed by the addition of 1.73 uM bradykinin like substrate by spectrofluorometry
- ChEMBL_40263 (CHEMBL653279) Concentration required to inhibit specific binding of [ 3H]BK (0.06 nM) to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%
- ChEMBL_40265 (CHEMBL656501) Concentration required to inhibit specific binding of [ 3H]BK (0.06 nM) to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_40436 (CHEMBL883337) In vitro binding affinity against Bradykinin receptor B2 in rat NG 108-15 neuroblastoma-glioma hybrid cell membranes using [3H]BK binding assay
- ChEMBL_423671 (CHEMBL912896) Activity at human bradykinin B1 receptor assessed as inhibition of Des-arg kallidin-induced increase of cytosolic calcium in CHO cells by FLIPR
- ChEMBL_748609 (CHEMBL1780462) Antagonist activity at human bradykinin B1 receptor expressed in CHO cells assessed as inhibition of agonist-induced calcium efflux by aquerin based assay
- ChEMBL_1550092 (CHEMBL3756038) Inhibition of Bacillus thermoproteolyticus thermolysin pretreated with compound for 1 hr followed by the addition of 3.33 uM bradykinin like substrate by spectrofluorometry assay
- ChEMBL_40264 (CHEMBL653280) Concentration required to inhibit specific binding of [3H]BK at 0.06 nM to Bradykinin receptor B2 in guinea pig ileum membrane preparations by 50%.
- ChEMBL_40286 (CHEMBL653418) Inhibition specific binding of [3H]BK (1.0 nM) to human Bradykinin receptor B2 which was expressed in CHO (Chinese hamster ovary) cells by 50%.
- ChEMBL_303777 (CHEMBL830134) Binding affinity of the [35S]- radiolabeled compound to human Bradykinin receptor B1 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_303778 (CHEMBL830135) Binding affinity of the [35S]- radiolabeled compound to human Bradykinin receptor B2 over-expressed in transgenic rats was determined by ex vivo receptor occupancy assay
- ChEMBL_40289 (CHEMBL653421) Concentration required to inhibit specific binding of [3H]BK (1.2 nM) to A-431 cells (human epidermoid carcinoma) which express Bradykinin receptor B2 by 50%.
- ChEMBL_40288 (CHEMBL653420) Concentration required to inhibit specific binding of [3H]BK at 1.2 nM to A-431 cells (human epidermoid carcinoma) which express Bradykinin receptor B2 by 50%.
- ChEMBL_429974 (CHEMBL917704) Antagonist activity at bradykinin B1 receptor expressed in CHO cells assessed as inhibition of des-arg10-kallidin-induced increase in cytosolic calcium level by FLIPR assay
- ChEMBL_797692 (CHEMBL1943912) Antagonist activity at human bradykinin B1 receptor expressed in CHO-D-/aequorin cells assessed as inhibition of DAK-induced intracellular calcium level after 1.5 to 2 hrs by luminometry analysis
- ChEMBL_1651749 (CHEMBL4001004) Antagonist activity against bradykinin B1 receptor in human HeLa cells assessed as inhibition of Des-Arg9-BK-induced increase in intracellular Ca2+ level by Fluro-4 direct calcium dye based fluorescence assay
- ChEMBL_1687679 (CHEMBL4038158) Inhibition of human plasma kallikrein spiked in pig vitreous using HMWK as substrate assessed as suppression of bradykinin release preincubated for 15 mins followed by substrate addition measured after 15 mins by ELISA
- ChEMBL_1687677 (CHEMBL4038156) Inhibition of plasma kallikrein in human plasma using HMWK as substrate assessed as suppression of kaolin-activated protein induced bradykinin release preincubated for 15 mins followed by substrate addition measured after 15 min by ELISA
- ChEMBL_863255 (CHEMBL2176198) Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substrate incubated for 15 mins prior to substrate addition measured after 15 mins by fluorimetric analysis
- Binding Assay For binding, Bradykinin-1 receptor antagonist compounds were added in various concentrations in 50 mM Tris pH 7.4, 5 mM MgCl2 together with 6 nM Kallidin (Des Arg10, Leu9), [3,4-Prolyl-3,4-3H(N)] (PerkinElmer, 1.85-4.44 TBq/mmol) to 40 μg membrane protein containing approximately 1 fmol Bradykinin-1 receptor and incubated for 15 min at 27° C. To determine non-specific binding 10 μM Lys-(Des-Arg9)-Bradykinin (Bachem) was added. Membranes were harvested through GF/B (glass fiber filter; PerkinElmer) plates, equilibrated with 0.5% polyethylenimine, air dried at 50° C. for 2 hr. Radioactivity was determined by counting in a topcounter (NXT Packard). Specific binding was defined as total binding minus nonspecific binding and typically represents about 90-95% of the total binding. Antagonist activity is expressed as Ki: inhibitor concentration required for 50% inhibition of specific binding corrected for the concentration of the radioligand.
- Inhibition Assay The activated cathepsin A was diluted in assay buffer (25 mM MES, pH 5.5, containing 5 mM DTT) and mixed with the test compound (dissolved in assay buffer containing (v/v) 3% DMSO) or, in the control experiments, with the vehicle in a multiple assay plate. After incubation for 15 min at room temperature, as substrate then bradykinin carrying an N-terminal Bodipy FL (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl) label (JPT Peptide Technologies GmbH; dissolved in assay buffer) was added to the mixture. The final concentration of cathepsin A was 833 ng/ml and the final concentration of labeled bradykinin 2 μM. After incubation for 15 min at room temperature the reaction was stopped by the addition of stop buffer (130 mM 2-(4-(2-hydroxy-ethyl)-piperazin-1-yl)-ethanesulfonic acid, pH 7.4, containing (v/v) 0.013% Triton X-100, 0.13% Coating Reagent 3 (Caliper Life Sciences), 6.5% DMSO and 20 μM ebelactone B (Sigma, #E0886)).
- Inhibition Assay The activated cathepsin A was diluted in assay buffer (25 mM MES, pH 5.5, containing 5 mM DTT) and mixed with the test compound (dissolved in assay buffer containing (v/v) 3% DMSO) or, in the control experiments, with the vehicle in a multiple assay plate. After incubation for 15 min at room temperature, as substrate then bradykinin carrying an N-terminal Bodipy FL (4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-propionyl) label (JPT Peptide Technologies GmbH; dissolved in assay buffer) was added to the mixture. The final concentration of cathepsin A was 833 ng/ml and the final concentration of labeled bradykinin 2 μM. After incubation for 15 min at room temperature the reaction was stopped by the addition of stop buffer (130 mM 2-(4-(2-hydroxy-ethyl)-piperazin-1-yl)-ethanesulfonic acid, pH 7.4, containing (v/v) 0.013%° Triton X-100, 0.13% Coating Reagent 3 (Caliper Life Sciences), 6.5% DMSO and 20 μM ebelactone B (Sigma, #E0886)).
- Calcium Flux Assays (hB1 IC50 IMR90) Notably, Compound Examples are tested in the FLIPR assays in the presence (hB1 free IC50) of 0.1% BSA in assay buffer, in order to assess the potency shifts due to serum protein binding of Compound Examples. The effect of BSA on the potency of endothelin receptor antagonists have been described in the prior art (Wu-Wong, J. R. et al. (1997), JPET 281: 791-798). The teaching can be applied in analogy to testing the potency of Bradykinin B1 receptor antagonist in the FLIPR assays. For the calcium flux assay, 80% confluent cells are detached from the culture vessels with Versene (Gibco), and seeded into 384-well plates (Cell binding Surface; Corning, N.Y.; #3683) at a density of 15,000 cells per well. Cells are seeded in a volume of 50 μL in medium without antibiotics and incubated overnight in a humidified atmosphere with 5% CO2 at 37° C. The following day, the medium is replaced with 20 μL of 5 μM Fluo-4AM dye (Molecular Probes) in assay buffer (2.5 mM probenicid, 1 mg/mL pluronic acid, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl, 1 mM MgCl2, 10 mM HEPES, 5.6 mM glucose, and 0.05% gelatine, pH 7.4), which contains or lacks 0.1% BSA for determination of compound potency units as hB1 IC50 or hB1 free IC50, respectively. The calcium indicator loaded cells are incubated at 37° C. for 2 hrs. Extracellular dye is then removed and each well is filled with 45 μL of assay buffer. Cell plates are kept in dark until used. Compound examples are assayed at 8 concentrations in triplicate. Serial 10-fold dilutions in 100% DMSO are made at a 100-times higher concentration than the final concentration, and then diluted 1:10 in assay buffer. 5 μL of each diluted compound is added to the well of cell plates (yielding final concentration with 1% DMSO), and incubated for 30 min at 28° C. before the addition of Bradykinin B1 receptor agonist on the FLIPR instrument. Agonist plates contain the agonist Lys-(Des-Arg)-Bradykinin (Bachem, Brackley) at 3.5×EC90 in assay buffer with 1% DMSO. The addition of agonist 20 μl per well to the assay plate is carried out on the FLIPR instrument while continuously monitoring Ca2+-dependent fluorescence at 538 nm. A peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin (Bachem, Brackley) at 20 □M is used to determine the full inhibition as control. Peak fluorescence is used to determine the response to agonist obtained at each concentration of Compound Examples by the following equation: % Response=100*(RFU(compound)−RFU(control))/(RFU(DMSO)−RFU(control)) RFU means relative fluorescence units. Control means full inhibition by the peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin at 20 □M.The response values are plotted against the logarithm of the compound concentrations. The Compound Examples are tested in triplicates per plate and mean values are plotted in Excel XLfit to determine IC50 values, percentage of maximal inhibition and the Hill slopes.
- Calcium Flux Assays (hB1 IC50 free) Notably, Compound Examples are tested in the FLIPR assays in the absence (hB1 free IC50) of 0.1% BSA in assay buffer, in order to assess the potency shifts due to serum protein binding of Compound Examples. The effect of BSA on the potency of endothelin receptor antagonists have been described in the prior art (Wu-Wong, J. R. et al. (1997), JPET 281: 791-798). The teaching can be applied in analogy to testing the potency of Bradykinin B1 receptor antagonist in the FLIPR assays. For the calcium flux assay, 80% confluent cells are detached from the culture vessels with Versene (Gibco), and seeded into 384-well plates (Cell binding Surface; Corning, N.Y.; #3683) at a density of 15,000 cells per well. Cells are seeded in a volume of 50 μL in medium without antibiotics and incubated overnight in a humidified atmosphere with 5% CO2 at 37° C. The following day, the medium is replaced with 20 μL of 5 μM Fluo-4AM dye (Molecular Probes) in assay buffer (2.5 mM probenicid, 1 mg/mL pluronic acid, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl, 1 mM MgCl2, 10 mM HEPES, 5.6 mM glucose, and 0.05% gelatine, pH 7.4), which contains or lacks 0.1% BSA for determination of compound potency units as hB1 IC50 or hB1 free IC50, respectively. The calcium indicator loaded cells are incubated at 37° C. for 2 hrs. Extracellular dye is then removed and each well is filled with 45 μL of assay buffer. Cell plates are kept in dark until used. Compound examples are assayed at 8 concentrations in triplicate. Serial 10-fold dilutions in 100% DMSO are made at a 100-times higher concentration than the final concentration, and then diluted 1:10 in assay buffer. 5 μL of each diluted compound is added to the well of cell plates (yielding final concentration with 1% DMSO), and incubated for 30 min at 28° C. before the addition of Bradykinin B1 receptor agonist on the FLIPR instrument. Agonist plates contain the agonist Lys-(Des-Arg)-Bradykinin (Bachem, Brackley) at 3.5×EC90 in assay buffer with 1% DMSO. The addition of agonist 20 μl per well to the assay plate is carried out on the FLIPR instrument while continuously monitoring Ca2+-dependent fluorescence at 538 nm. A peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin (Bachem, Brackley) at 20 □M is used to determine the full inhibition as control. Peak fluorescence is used to determine the response to agonist obtained at each concentration of Compound Examples by the following equation: % Response=100*(RFU(compound)−RFU(control))/(RFU(DMSO)−RFU(control)) RFU means relative fluorescence units. Control means full inhibition by the peptide antagonist Lys-(Des-Arg-Leu)-Bradykinin at 20 □M.The response values are plotted against the logarithm of the compound concentrations. The Compound Examples are tested in triplicates per plate and mean values are plotted in Excel XLfit to determine IC50 values, percentage of maximal inhibition and the Hill slopes.
 CAS_58-82-2 NSC_105044 Bradykinin BDBM82076
CAS_58-82-2 NSC_105044 Bradykinin BDBM82076 Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35
Galanin-(1-13)-bradykinin-(2-9)-amide Galanin (1-13)-bradikinin(2-9) CHEMBL503473 BDBM50273370 GWTLNSAGYLLGPPPGFSPFR-CONH2 M35 (bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)
(bradykinin triacetate)2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid (BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH CHEMBL406291 bradykinin BDBM50049949 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid 2-(2-{[1-(2-{2-[2-({1-[1-(2-Amino-5-guanidino-pentanoyl)-pyrrolidine-2-carbonyl]-pyrrolidine-2-carbonyl}-amino)-acetylamino]-3-phenyl-propionylamino}-3-hydroxy-propionyl)-pyrrolidine-2-carbonyl]-amino}-3-phenyl-propionylamino)-5-guanidino-pentanoic acid(Bradykinin)