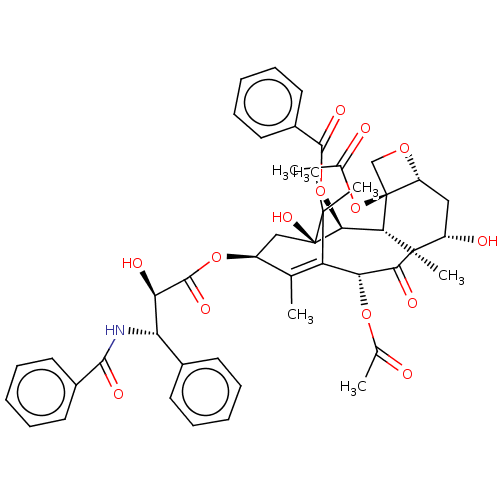
 taxol CHEMBL428647 BDBM50001839 PACLITAXEL
taxol CHEMBL428647 BDBM50001839 PACLITAXEL BDBM573947 US11453697, Example 132 2-amino-9-[(5S,7R,8R,12aR,14R,15S,15aR)- 14-(6-amino-9H-purin-9-yl)-15-fluoro-2,10- dihydroxy-2,10-disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-oe (Diastereomer 3)
BDBM573947 US11453697, Example 132 2-amino-9-[(5S,7R,8R,12aR,14R,15S,15aR)- 14-(6-amino-9H-purin-9-yl)-15-fluoro-2,10- dihydroxy-2,10-disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-oe (Diastereomer 3)
- Dong, Y; Wang, S; Wang, C; Li, Z; Ma, Y; Liu, G Antagonizing NOD2 Signaling with Conjugates of Paclitaxel and Muramyl Dipeptide Derivatives Sensitizes Paclitaxel Therapy and Significantly Prevents Tumor Metastasis. J Med Chem 60: 1219-1224 (2017)
- Machulkin, AE; Skvortsov, DA; Ivanenkov, YA; Ber, AP; Kavalchuk, MV; Aladinskaya, AV; Uspenskaya, AA; Shafikov, RR; Plotnikova, EA; Yakubovskaya, RI; Nimenko, EA; Zyk, NU; Beloglazkina, EK; Zyk, NV; Koteliansky, VE; Majouga, AG Synthesis and biological evaluation of PSMA-targeting paclitaxel conjugates. Bioorg Med Chem Lett 29: 2229-2235 (2019)
- Chen, YF; Wu, CH; Chen, LH; Lee, HW; Lee, JC; Yeh, TK; Chang, JY; Chou, MC; Wu, HL; Lai, YP; Song, JS; Yeh, KC; Chen, CT; Lee, CJ; Shia, KS; Shen, MR Discovery of Potential Neuroprotective Agents against Paclitaxel-Induced Peripheral Neuropathy. J Med Chem 65: 4767-4782 (2022)
- Day, CL; Smits, C; Fan, FC; Lee, EF; Fairlie, WD; Hinds, MG Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol 380: 958-71 (2008)
- Lis, LG; Smart, MA; Luchniak, A; Gupta, ML; Gurvich, VJ Synthesis and Biological Evaluation of a Biotinylated Paclitaxel With an Extra-Long Chain Spacer Arm. ACS Med Chem Lett 3: 745-748 (2012)
- Ayoub, AT; Abou El-Magd, RM; Xiao, J; Lewis, CW; Tilli, TM; Arakawa, K; Nindita, Y; Chan, G; Sun, L; Glover, M; Klobukowski, M; Tuszynski, J Antitumor Activity of Lankacidin Group Antibiotics Is Due to Microtubule Stabilization via a Paclitaxel-like Mechanism. J Med Chem 59: 9532-9540 (2016)
- Poh Yen, K; Stanslas, J; Zhang, T; Li, H; Wang, X; Kok Meng, C; Kok Wai, L Synthesis of small molecules targeting paclitaxel-induced MyD88 expression in triple-negative breast cancer cell lines. Bioorg Med Chem 49: (2021)
- Guo, XM; Yadav, MB; Khan, M; Hao, CW; Lin, CY; Huang, T; Wu, J; Fan, BM; Bian, ZX Bradykinin-Potentiating Peptide-Paclitaxel Conjugate Directed at Ectopically Expressed Angiotensin-Converting Enzyme in Triple-Negative Breast Cancer. J Med Chem 64: 17051-17062 (2021)
- Kawahara, B; Faull, KF; Janzen, C; Mascharak, PK Carbon Monoxide Inhibits Cytochrome P450 Enzymes CYP3A4/2C8 in Human Breast Cancer Cells, Increasing Sensitivity to Paclitaxel. J Med Chem 64: 8437-8446 (2021)
- Iwaki, Y; Ohhata, A; Nakatani, S; Hisaichi, K; Okabe, Y; Hiramatsu, A; Watanabe, T; Yamamoto, S; Nishiyama, T; Kobayashi, J; Hirooka, Y; Moriguchi, H; Maeda, T; Katoh, M; Komichi, Y; Ota, H; Matsumura, N; Okada, M; Sugiyama, T; Saga, H; Imagawa, A ONO-8430506: A Novel Autotaxin Inhibitor That Enhances the Antitumor Effect of Paclitaxel in a Breast Cancer Model. ACS Med Chem Lett 11: 1335-1341 (2020)
- Chen, X; Plasencia, C; Hou, Y; Neamati, N Synthesis and biological evaluation of dimeric RGD peptide-paclitaxel conjugate as a model for integrin-targeted drug delivery. J Med Chem 48: 1098-106 (2005)
- Dadgar, S; Ramjan, Z; Floriano, WB Paclitaxel is an inhibitor and its boron dipyrromethene derivative is a fluorescent recognition agent for botulinum neurotoxin subtype A. J Med Chem 56: 2791-803 (2013)
- Kimura, Y; Aoki, J; Kohno, M; Ooka, H; Tsuruo, T; Nakanishi, O P-glycoprotein inhibition by the multidrug resistance-reversing agent MS-209 enhances bioavailability and antitumor efficacy of orally administered paclitaxel. Cancer Chemother Pharmacol 49: 322-8 (2002)
- Shi, W; Zhang, P; Zou, F; Zhou, J; Yin, Z; Cai, Z; Ghaleb, H; Jiang, Y; Huang, W; Liu, Y; Qiu, Q; Qian, H Exploration of novel phthalazinone derivatives as potential efflux transporter inhibitors for reversing multidrug resistance and improving the oral absorption of paclitaxel. Eur J Med Chem 233: (2022)
- Colombo, R; Mingozzi, M; Belvisi, L; Arosio, D; Piarulli, U; Carenini, N; Perego, P; Zaffaroni, N; De Cesare, M; Castiglioni, V; Scanziani, E; Gennari, C Synthesis and biological evaluation (in vitro and in vivo) of cyclic arginine-glycine-aspartate (RGD) peptidomimetic-paclitaxel conjugates targeting integrinaVß3. J Med Chem 55: 10460-74 (2012)
- Wang, S; Yang, J; Li, X; Liu, Z; Wu, Y; Si, G; Tao, Y; Zhao, N; Hu, X; Ma, Y; Liu, G Discovery of 1,4-Benzodiazepine-2,5-dione (BZD) Derivatives as Dual Nucleotide Binding Oligomerization Domain Containing 1/2 (NOD1/NOD2) Antagonists Sensitizing Paclitaxel (PTX) To Suppress Lewis Lung Carcinoma (LLC) Growth in Vivo. J Med Chem 60: 5162-5192 (2017)
- Moser, PC; Bergis, OE; Jegham, S; Lochead, A; Duconseille, E; Terranova, JP; Caille, D; Berque-Bestel, I; Lezoualc'h, F; Fischmeister, R; Dumuis, A; Bockaert, J; George, P; Soubrié, P; Scatton, B J Pharmacol Exp Ther 302: 731-41 (2002)
- Machulkin, AE; Uspenskaya, AA; Zyk, NU; Nimenko, EA; Ber, AP; Petrov, SA; Polshakov, VI; Shafikov, RR; Skvortsov, DA; Plotnikova, EA; Pankratov, AA; Smirnova, GB; Borisova, YA; Pokrovsky, VS; Kolmogorov, VS; Vaneev, AN; Khudyakov, AD; Chepikova, OE; Kovalev, S; Zamyatnin, AA; Erofeev, A; Gorelkin, P; Beloglazkina, EK; Zyk, NV; Khazanova, ES; Majouga, AG J Med Chem 64: 17123-17145 (2021)
- Zindo, FT; Malan, SF; Omoruyi, SI; Enogieru, AB; Ekpo, OE; Joubert, J Eur J Med Chem 163: 83-94 (2019)
- Hutt, OE; Saubern, S; Winkler, DA Bioorg Med Chem 19: 5903-11 (2011)
- Eldehna, WM; Abo-Ashour, MF; Nocentini, A; Gratteri, P; Eissa, IH; Fares, M; Ismael, OE; Ghabbour, HA; Elaasser, MM; Abdel-Aziz, HA; Supuran, CT Eur J Med Chem 139: 250-262 (2017)
- Narwal, M; Koivunen, J; Haikarainen, T; Obaji, E; Legala, OE; Venkannagari, H; Joensuu, P; Pihlajaniemi, T; Lehtiö, L J Med Chem 56: 7880-9 (2013)
- Bogen, SL; Arasappan, A; Bennett, F; Chen, K; Jao, E; Liu, YT; Lovey, RG; Venkatraman, S; Pan, W; Parekh, T; Pike, RE; Ruan, S; Liu, R; Baroudy, B; Agrawal, S; Chase, R; Ingravallo, P; Pichardo, J; Prongay, A; Brisson, JM; Hsieh, TY; Cheng, KC; Kemp, SJ; Levy, OE; Lim-Wilby, M; Tamura, SY; Saksena, AK; Girijavallabhan, V; Njoroge, FG J Med Chem 49: 2750-7 (2006)
- Holmes, IP; Blunt, RJ; Lorthioir, OE; Blowers, SM; Gribble, A; Payne, AH; Stansfield, IG; Wood, M; Woollard, PM; Reavill, C; Howes, CM; Micheli, F; Di Fabio, R; Donati, D; Terreni, S; Hamprecht, D; Arista, L; Worby, A; Watson, SP Bioorg Med Chem Lett 20: 2013-6 (2010)
- Koval, VS; Arutyunyan, AF; Salyanov, VI; Kostyukov, AA; Melkina, OE; Zavilgelsky, GB; Klimova, RR; Kushch, AA; Korolev, SP; Agapkina, YY; Gottikh, MB; Vaiman, AV; Rybalkina, EY; Susova, OY; Zhuze, AL Bioorg Med Chem 28: (2020)
- Ellis, ES; Byrne, C; Murphy, OE; Tilford, NS; Baxter, GS Br J Pharmacol 114: 400-4 (1995)
- Ozaki, S; Oe, H; Kitamura, S J Nat Prod 71: 981-4 (2008)
- Ishibuchi, S; Morimoto, H; Oe, T; Ikebe, T; Inoue, H; Fukunari, A; Kamezawa, M; Yamada, I; Naka, Y Bioorg Med Chem Lett 11: 879-82 (2001)
- Harada, K; Mizukami, J; Kadowaki, S; Matsuda, I; Watanabe, T; Oe, Y; Kodama, Y; Aoki, K; Suwa, K; Fukuda, S; Yata, S; Inaba, T Bioorg Med Chem Lett 28: 1228-1233 (2018)
- ChEMBL_839291 (CHEMBL2077962) TP_TRANSPORTER: drug resistance (paclitaxel) in HCT15/CL02 cells
- ChEMBL_1896435 (CHEMBL4398470) Inhibition of Bcl2 (unknown origin) after 2 hrs by fluorescein-PUMA based fluorescence polarization assay
- ChEMBL_1896436 (CHEMBL4398471) Inhibition of Bcl-xL (unknown origin) after 2 hrs by fluorescein-PUMA based fluorescence polarization assay
- ChEMBL_1487657 (CHEMBL3532643) Reversible inhibition of human CYP2C8 paclitaxel 6alpha-hydroxylase activity
- ChEMBL_1904909 (CHEMBL4407267) Inhibition of CYP2C8 (unknown origin) using paclitaxel as substrate
- ChEMBL_838757 (CHEMBL2078583) TP_TRANSPORTER: drug resistance (paclitaxel) in MES-SA/DX5 cells
- ChEMBL_1827556 (CHEMBL4327430) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM
- ChEMBL_2116914 (CHEMBL4825980) Inhibition of paclitaxel stimulated- P-gp ATPase activity (unknown origin)
- ChEMBL_2116923 (CHEMBL4825989) Inhibition of P-gp (unknown origin) expressed in MCF7 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring reduction in paclitaxel EC50 at 50 nM incubated for 72 hrs in presence of paclitaxel by SRB assay (Rvb = 11.5 nM)
- ChEMBL_2151595 (CHEMBL5036057) Inhibition of CYP2C8 in human liver microsome using paclitaxel as substrate
- ChEMBL_2024838 (CHEMBL4678651) Inhibition of ABCB1 in multidrug-resistant human SW620/Ad300 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 4.23 +/- 0.6 uM)
- ChEMBL_2116924 (CHEMBL4825990) Inhibition of P-gp (unknown origin) expressed in MES-SA/DX5 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring reduction in paclitaxel EC50 at 50 nM incubated for 72 hrs in presence of paclitaxel by SRB assay (Rvb = 294.6 nM)
- ChEMBL_2444934 Inhibition of P-gp in human Caco-2 cells in presence of paclitaxel
- ChEMBL_2024816 (CHEMBL4678629) Inhibition of ABCB1 (unknown origin) expressed in HEK293 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 4 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 91.40 +/- 23.32 nM)
- ChEMBL_2024817 (CHEMBL4678630) Inhibition of ABCB1 (unknown origin) expressed in HEK293 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 20 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 91.40 +/- 23.32 nM)
- ChEMBL_2024818 (CHEMBL4678631) Inhibition of ABCB1 (unknown origin) expressed in HEK293 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 91.40 +/- 23.32 nM)
- ChEMBL_2024829 (CHEMBL4678642) Inhibition of ABCB1 in multidrug-resistant human KB-C2 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 4 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 1886.37 +/- 243.05 nM)
- ChEMBL_2024830 (CHEMBL4678643) Inhibition of ABCB1 in multidrug-resistant human KB-C2 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 20 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 1886.37 +/- 243.05 nM)
- ChEMBL_2024834 (CHEMBL4678647) Inhibition of ABCB1 in multidrug-resistant human SW620/Ad300 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 4 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 4233.18 +/- 499.10 nM)
- ChEMBL_2024835 (CHEMBL4678648) Inhibition of ABCB1 in multidrug-resistant human SW620/Ad300 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 20 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 4233.18 +/- 499.10 nM)
- ChEMBL_2024836 (CHEMBL4678649) Inhibition of ABCB1 in multidrug-resistant human SW620/Ad300 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 4233.18 +/- 499.10 nM)
- ChEMBL_2024839 (CHEMBL4678652) Inhibition of ABCB1 in multidrug-resistant human KB-C2 cells assessed as increase in reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay (Rvb = 1886.37 +/- 243.05 nM)
- ChEMBL_1587229 (CHEMBL3825965) Inhibition of ABCB1 in human KBVIN cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 8 uM after 72 hrs by SRB assay (Rvb = 1196.09 +/- 44.8 nM)
- ChEMBL_1587230 (CHEMBL3825966) Inhibition of ABCB1 in human KBVIN cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by SRB assay (Rvb = 1196.09 +/- 44.8 nM)
- ChEMBL_1755244 (CHEMBL4190004) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 1 uM after 4 days by CellTiter-Glo assay (Rvb = 1202 nM)
- ChEMBL_1755245 (CHEMBL4190005) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 3 uM after 4 days by CellTiter-Glo assay (Rvb = 1202 nM)
- ChEMBL_1755246 (CHEMBL4190006) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 10 uM after 4 days by CellTiter-Glo assay (Rvb = 1202 nM)
- ChEMBL_1755247 (CHEMBL4190007) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 20 uM after 4 days by CellTiter-Glo assay (Rvb = 1202 nM)
- ChEMBL_1755248 (CHEMBL4190008) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of paclitaxel-mediated cytotoxicity by measuring paclitaxel IC50 at 30 uM after 4 days by CellTiter-Glo assay (Rvb = 1202 nM)
- ChEMBL_496041 (CHEMBL998471) Displacement of [3H]paclitaxel from pig biotinylated tubulin after 2 hrs by SPA
- ChEMBL_1741905 (CHEMBL4157655) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 3.39 +/- 0.84 microM)
- ChEMBL_1741906 (CHEMBL4157656) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 20 uM after 72 hrs by MTT assay (Rvb = 3.39 +/- 0.84 microM)
- ChEMBL_1741907 (CHEMBL4157657) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 4 uM after 72 hrs by MTT assay (Rvb = 3.39 +/- 0.84 microM)
- ChEMBL_1741941 (CHEMBL4157691) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 0.09 +/- 0.02 microM)
- ChEMBL_1741942 (CHEMBL4157692) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 20 uM after 72 hrs by MTT assay (Rvb = 0.09 +/- 0.02 microM)
- ChEMBL_1741943 (CHEMBL4157693) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 4 uM after 72 hrs by MTT assay (Rvb = 0.09 +/- 0.02 microM)
- ChEMBL_2089947 (CHEMBL4771210) Inhibition of ABCB1 in FLp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 2.5 uM measured after 72 hrs by SRB assay (Rvb = 11.8 +/- 1.13 nM)
- ChEMBL_2089948 (CHEMBL4771211) Inhibition of ABCB1 in FLp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by SRB assay (Rvb = 11.8 +/- 1.13 nM)
- ChEMBL_2089959 (CHEMBL4771222) Inhibition of ABCB1 in human HeLa S3 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 2.5 uM measured after 72 hrs by SRB assay (Rvb = 26.50 +/- 4.1 nM)
- ChEMBL_2089960 (CHEMBL4771223) Inhibition of ABCB1 in human HeLa S3 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by SRB assay (Rvb = 26.50 +/- 4.1 nM)
- ChEMBL_2089965 (CHEMBL4771228) Inhibition of ABCB1 in human KB-VIN cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 2.5 uM measured after 72 hrs by SRB assay (Rvb = 1762 +/- 63 nM)
- ChEMBL_2089966 (CHEMBL4771229) Inhibition of ABCB1 in human KB-VIN cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by SRB assay (Rvb = 1762 +/- 63 nM)
- ChEMBL_2151092 (CHEMBL5035554) Inhibition of P-glycoprotein in human LCC6MDR cells assessed as reversal fold by measuring reduction in paclitaxel by measuring paclitaxel IC50 at 1 uM after 5 days by Cell Titer-Glo luminescence assay
- ChEMBL_1668632 (CHEMBL4018520) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 mins
- ChEMBL_874592 (CHEMBL2186228) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins
- ChEMBL_1500054 (CHEMBL3584050) Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for paclitaxel at 1 uM after 5 days by CellTiter 96 Aqueous assay
- ChEMBL_1500167 (CHEMBL3584716) Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversible of paclitaxel resistance measured as IC50 for paclitaxel at 10 uM after 5 days by CellTiter 96 Aqueous assay
- ChEMBL_1827565 (CHEMBL4327439) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 24 hrs by MTT assay (Rvb = 3837.57 uM)
- ChEMBL_1827566 (CHEMBL4327440) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 24 hrs by MTT assay (Rvb = 3837.57 uM)
- ChEMBL_1827569 (CHEMBL4327443) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 48 hrs by MTT assay (Rvb = 1396.57 uM)
- ChEMBL_1827570 (CHEMBL4327444) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 48 hrs by MTT assay (Rvb = 1396.57 uM)
- ChEMBL_2111356 (CHEMBL4820206) Reversal of P-glycoprotein mediated multidrug resistance in human KBV cells assessed as reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by MTT assay
- ChEMBL_2111357 (CHEMBL4820207) Reversal of P-glycoprotein mediated multidrug resistance in human KBV cells assessed as reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM measured after 72 hrs by MTT assay
- ChEMBL_1877072 (CHEMBL4378466) Displacement of fluorescein labelled Puma-BH3 peptide from His-tagged-TEV fused human Mcl-1 incubated for 2 hrs by fluorescence polarization assay
- ChEMBL_1826942 (CHEMBL4326816) Reversal of P-gp-mediated multidrug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 72 hrs by MTT assay (Rvb = 1353.98 +/- 303.33 nM)
- ChEMBL_1826943 (CHEMBL4326817) Reversal of P-gp-mediated multidrug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 1353.98 +/- 303.33 nM)
- ChEMBL_1827560 (CHEMBL4327434) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 398.34 +/- 0.58 uM)
- ChEMBL_1827561 (CHEMBL4327435) Reversal of P-gp-mediated drug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 72 hrs by MTT assay (Rvb = 398.34 +/- 0.58 uM)
- ChEMBL_1862982 (CHEMBL4363838) Reversal of P-gp-mediated multidrug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 72 hrs by MTT assay (Rvb = 352.31 +/- 10.9 nM)
- ChEMBL_1862983 (CHEMBL4363839) Reversal of P-gp-mediated multidrug resistance in human KBV cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 352.31 +/- 10.9 nM)
- ChEMBL_2111360 (CHEMBL4820210) Reversal of P-glycoprotein mediated multidrug resistance in human MCF-7T cells assessed as reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by MTT assay
- ChEMBL_2111361 (CHEMBL4820211) Reversal of P-glycoprotein mediated multidrug resistance in human MCF-7T cells assessed as reversal of resistance to paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM measured after 72 hrs by MTT assay
- ChEMBL_2135225 (CHEMBL4844835) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as reduction in paclitaxel IC50 at 0.5 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay
- ChEMBL_2135226 (CHEMBL4844836) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as reduction in paclitaxel IC50 at 1 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay
- ChEMBL_2135227 (CHEMBL4844837) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as reduction in paclitaxel IC50 at 2 uM pre-incubated for 4 hrs followed by paclitaxel addition and measured after 72 hrs by MTT assay
- ChEMBL_1508932 (CHEMBL3602151) Modulation of P-gp in human MDA435/LCC6MDR cells assessed as reversal of paclitaxel resistance
- ChEMBL_1864328 (CHEMBL4365303) Inhibition of Pgp (unknown origin) expressed in MDCK cells assessed as reduction in paclitaxel transport
- ChEMBL_572591 (CHEMBL1025207) Inhibition of CYP2C8 in human liver microsomes assessed as inhibition of paclitaxel 6-alpha-hydroxylation
- ChEMBL_854489 (CHEMBL2160811) Inhibition of human CYP2C8 in liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 48 hrs
- ChEMBL_1587212 (CHEMBL3825948) Inhibition of full length human ABCB1 expressed in Flp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 8 uM after 72 hrs by SRB assay (Rvb = 644.78 +/- 2.6 nM)
- ChEMBL_1587213 (CHEMBL3825949) Inhibition of full length human ABCB1 expressed in Flp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 72 hrs by SRB assay (Rvb = 644.78 +/- 2.6 nM)
- ChEMBL_2150563 (CHEMBL5035025) Binding affinity to human Bcl-2 assessed as inhibition constant incubated for 2 hrs in presence of fluorescein-PUMA peptide by fluorescence polarization assay
- ChEMBL_2150564 (CHEMBL5035026) Binding affinity to human Bcl-xl assessed as inhibition constant incubated for 2 hrs in presence of fluorescein-PUMA peptide by fluorescence polarization assay
- ChEMBL_2184282 (CHEMBL5096364) Binding affinity to human Bcl-2 assessed as inhibition constant incubated for 2 hrs in presence of fluorescein-PUMA peptide by fluorescence polarization assay
- ChEMBL_2184283 (CHEMBL5096365) Binding affinity to human Bcl-xL assessed as inhibition constant incubated for 2 hrs in presence of fluorescein-PUMA peptide by fluorescence polarization assay
- H6PD Activity Assay HEK293 H6PD-OE and HEK293 pellets were lysed and the lysate was quantified with the Bradford protein assay. Reaction mix containing galactose-6-phosphate (Gal6P) was combined with increasing concentrations of PARPi or DMSO. As an additional control, reaction mix without substrate was combined with HEK293 H6PD-OE lysate. Lysate from HEK293 or HEK293 H6PD-OE was added to each well to start the reactions. The absorbance at 340 nM (NADPH) was measured for 90 min at 10 min intervals. The 60 min data, which were within the linear range, was used for analysis. Background absorbance was subtracted and the H6PD activity of theHEK293 H6PD-OE lysates treated with PARPi was normalized to the DMSO control.
- ChEMBL_2089953 (CHEMBL4771216) Inhibition of human full-length ABCB1 expressed in FLp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 2.5 uM measured after 72 hrs by SRB assay (Rvb = 797 +/- 73 nM)
- ChEMBL_2089954 (CHEMBL4771217) Inhibition of human full-length ABCB1 expressed in FLp-In-293 cells assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM measured after 72 hrs by SRB assay (Rvb = 797 +/- 73 nM)
- ChEMBL_1501739 (CHEMBL3587719) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate by LC-MS/MS analysis
- ChEMBL_1655299 (CHEMBL4004665) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 15 mins
- ChEMBL_2156137 (CHEMBL5040797) Inhibition of CYP2C8 in human liver microsomes using Paclitaxel as substrate by LC-MS/MS analysis
- ChEMBL_738490 (CHEMBL1743391) Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes
- ChEMBL_738491 (CHEMBL1743392) Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using human liver microsomes
- ChEMBL_738492 (CHEMBL1743393) Mechanism based inhibition of human cytochrome P450 2C8 measured by paclitaxel hydroxylation using a recombinant system
- ChEMBL_850903 (CHEMBL2156886) Inhibition of human CYP2C8 using paclitaxel as substrate after 45 mins by LC/MS/MS analysis
- ChEMBL_1855736 (CHEMBL4356465) Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 3 uM after 48 hrs by MTT assay (Rvb = 2120 nM)
- ChEMBL_1855745 (CHEMBL4356474) Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 5 uM after 48 hrs by MTT assay (Rvb = 2120 nM)
- ChEMBL_1855746 (CHEMBL4356475) Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 7 uM after 48 hrs by MTT assay (Rvb = 2120 nM)
- ChEMBL_1855747 (CHEMBL4356476) Reversal of P-gp mediated multidrug resistance in human DU145-TxR cells overexpressing P-gp assessed as potentiation of paclitaxel-induced cytotoxicity by measuring paclitaxel IC50 at 10 uM after 48 hrs by MTT assay (Rvb = 2120 nM)
- ChEMBL_1877087 (CHEMBL4378481) Displacement of fluorescein labelled Puma-BH3 peptide from human BCl2 expressed in Escherichia coli BL21 (DE3) pLysS cells incubated for 2 hrs by fluorescence polarization assay
- ChEMBL_1284966 (CHEMBL3108547) Modulation of p-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversal of paclitaxel resistance
- ChEMBL_1493770 (CHEMBL3530853) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 8 mins by LC-MS/MS analysis
- ChEMBL_1616316 (CHEMBL3858385) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 15 mins by LC/MS/MS analysis
- ChEMBL_1703503 (CHEMBL4054736) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 15 mins by LC-MS/MS analysis
- ChEMBL_1932999 (CHEMBL4478651) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 10 mins by LC/MS/MS analysis
- ChEMBL_2066470 (CHEMBL4721723) Inhibition of human liver microsome CYP2C8 using paclitaxel as substrate incubated for 20 mins by LC-MS/MS analysis
- ChEMBL_977241 (CHEMBL2416912) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 20 mins by LC-MS analysis
- ChEMBL_1877076 (CHEMBL4378470) Displacement of fluorescein labelled Puma-BH3 peptide from C-terminal MBP-fused human Mcl-1 (173 to 321 residues) expressed in Escherichia coli BL21(DE3)pLysS by fluorescence polarization assay
- ChEMBL_1281756 (CHEMBL3100525) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 20 mins by LC-MS/MS analysis
- ChEMBL_813100 (CHEMBL2020719) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins by LC-MS/MS analysis
- ChEMBL_1873795 (CHEMBL4375084) Displacement of N-terminal fluorescein labelled Puma-BH3 peptide from TEV-fused His-tagged human Mcl-1 expressed in Escherichia coliBL21(DE3) pLysS cells after 2 hrs by fluorescence polarization assay
- ChEMBL_1487046 (CHEMBL3531872) Competitive inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis
- ChEMBL_1668634 (CHEMBL4018522) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 2 to 40 mins measured after 2 mins
- ChEMBL_993293 (CHEMBL2443950) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 5 to 60 mins by LC-MS/MS analysis
- ChEMBL_1873796 (CHEMBL4375085) Displacement of N-terminal fluorescein labelled Puma-BH3 peptide from human N-terminal thrombin cleavage site-fused/His6-tagged Bcl-2 expressed in Escherichia coliBL21(DE3) pLysS cells by fluorescence polarization assay
- ChEMBL_1487039 (CHEMBL3531865) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 5 to 30 mins by LC-MS/MS analysis
- ChEMBL_1487047 (CHEMBL3532217) Linear mixed inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6-hydroxylation after 20 mins by LC-MS/MS analysis
- ChEMBL_1755242 (CHEMBL4190002) Inhibition of ALDH1A1 in human SKOV3TR cells assessed as potentiation of 100 nM paclitaxel-mediated cytotoxicity after 4 days by CellTiter-Glo assay
- ChEMBL_2150562 (CHEMBL5035024) Binding affinity to full length human MCL1 (173 to 321 residues) expressed in Escherichia coli BL21 (DE3) assessed as inhibition constant incubated for 2 hrs in presence of fluorescein-PUMA peptide by fluorescence polarization assay
- ChEMBL_1492464 (CHEMBL3528839) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPH
- ChEMBL_1492465 (CHEMBL3528840) Inhibition of CYP2C9 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 3 mins by LC-MS/MS analysis in presence of NADPH
- ChEMBL_1822761 (CHEMBL4322525) Inhibition of P-gp (unknown origin) expressed in human LCC6MDR cells assessed as reversal of paclitaxel resistance after 5 days by MTS/PMS assay
- ChEMBL_1839489 (CHEMBL4339704) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate measured after 15 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_1904558 (CHEMBL4406780) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate incubated for 40 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_1992478 (CHEMBL4626213) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate incubated for 20 mins in presence of NADPH by LC/MS/MS analysis
- ChEMBL_2101711 (CHEMBL4810107) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins followed by NADPH addition by LC-MS/MS analysis
- ChEMBL_2119410 (CHEMBL4828476) Inhibition of P-gp (unknown origin) expressed in human LCC6MDR cells assessed as reversal of paclitaxel resistance after 5 days by MTS/PMS assay
- ChEMBL_1492474 (CHEMBL3528849) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of NADPH
- ChEMBL_1492475 (CHEMBL3528850) Inhibition of CYP2C9 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation preincubated for 15 mins by LC-MS/MS analysis in presence of NADPH
- ChEMBL_1661001 (CHEMBL4010613) Inhibition of human CYP2C8 using paclitaxel as substrate incubated for 5 mins followed by NADPH addition measured after 30 mins by LC-MS/MS analysis
- ChEMBL_2253560 (CHEMBL5167770) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate incubated for 5 to 45 mins in presence of NADPH by LC/MS analysis
- ChEMBL_1677530 (CHEMBL4027673) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate upto 10 uM after 20 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_2124311 (CHEMBL4833544) Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6a-hydroxylation reaction incubated for 30 mins in presence of NADP by LC-MS/MS analysis
- ChEMBL_1500056 (CHEMBL3584052) Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as reversing paclitaxel resistance measured as cell survival after 5 days by MTS assay
- ChEMBL_1668633 (CHEMBL4018521) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 2 to 40 mins followed by NADPH-generating system addition measured after 2 mins
- ChEMBL_2547700 Inhibition of Mps1 in human A2780 cells assessed as abolish in KNL-1 phosphorylation at Thr-875 level and pretreated with 100 nmol/L paclitaxel within 30 mins
- ChEMBL_1764605 (CHEMBL4199852) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate pretreated for 5 mins followed by NADPH addition and measured after 10 mins by LC-MS analysis
- ChEMBL_2212604 (CHEMBL5125553) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins followed by NADPH addition measured after 10 mins by LC-MS/MS analysis
- ChEMBL_1487322 (CHEMBL3535114) Inhibition of CYP2J2 in human liver microsomes using 7 probe cocktail containing phenacetin, paclitaxel, diclofenac, S-mephenytoin, dextromethorphan, astemizole and midazolam after 8 mins by LC-MS/MS analysis
- ChEMBL_1508927 (CHEMBL3602146) Modulation of P-gp (unknown origin) transfected in human MDA435/LCC6MDR cells assessed as paclitaxel IC50 for cell growth inhibition at 1 uM after 5 days by MTS assay
- ChEMBL_1668631 (CHEMBL4018519) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 30 mins in presence of NADPH-generating system followed by substrate addition measured after 5 mins
- ChEMBL_2116922 (CHEMBL4825988) Inhibition of P-gp (unknown origin) expressed in MES-SA/DX5 cells assessed as cell growth inhibition after 3 days in presence of 200 nM paclitaxel by MTT assay
- ChEMBL_2116946 (CHEMBL4826012) Inhibition of P-gp in human Caco-2 cells assessed as reduction in paclitaxel efflux pre-incubated for 30 mins and measured after 120 mins by LC-MS/MS analysis
- ChEMBL_2330581 Negative allosteric modulator activity at P-gp in human LCC6MDR cells overexpressing P-gp assessed as reversal of P-gp mediated paclitaxel resistance activity incubated for 5 days by MTS assay
- ChEMBL_2296574 Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate assessed as reduction in substrate hydroxylation incubated for 5 mins in presence of beta-NADPH measured by LC-MS/MS analysis
- ChEMBL_2296584 Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate assessed as reduction in substrate hydroxylation incubated for 30 mins in presence of beta-NADPH measured by LC-MS/MS analysis
- ChEMBL_1660963 (CHEMBL4010575) Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 20 mins followed by substrate addition measured after 30 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_2330580 Negative allosteric modulator activity at P-gp in human LCC6MDR cells overexpressing P-gp assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay
- ChEMBL_1660900 (CHEMBL4010512) Inhibition of CYP2C8 in human liver microsomes assessed as enzyme-mediated metabolite formation using paclitaxel as substrate incubated for 5 mins followed by NADPH addition measured after 30 mins by LC-MS/MS analysis
- ChEMBL_2330597 Negative allosteric modulator activity at human wildtype P-gp expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 540.5 +/- 20.3 nM)
- ChEMBL_2330596 Negative allosteric modulator activity at human P-gp G1114A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 5.6 +/- 1.1 nM)
- ChEMBL_2330598 Negative allosteric modulator activity at human P-gp I1115A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 7.5 +/- 2.4 nM)
- ChEMBL_2330599 Negative allosteric modulator activity at human P-gp H1195A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 3.4 +/- 1.5 nM)
- ChEMBL_2330600 Negative allosteric modulator activity at human P-gp T1226A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 6.3 +/- 2.1 nM)
- ChEMBL_2330601 Negative allosteric modulator activity at human P-gp Q1193A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 483.4 +/- 17.2 nM)
- ChEMBL_2330602 Negative allosteric modulator activity at human P-gp L1113A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 590 +/- 14.2 nM)
- ChEMBL_2330603 Negative allosteric modulator activity at human P-gp C1227A mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 352.1 +/- 30.6 nM)
- ChEMBL_2330604 Negative allosteric modulator activity at human P-gp Q1193F mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 453.6 +/- 14.8 nM)
- ChEMBL_2330605 Negative allosteric modulator activity at human P-gp Q1193E mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 395.8 +/- 13.4 nM)
- ChEMBL_2330606 Negative allosteric modulator activity at human P-gp Q1193K mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 466.2 +/- 72.3 nM)
- ChEMBL_2330607 Negative allosteric modulator activity at human P-gp Q1193C mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 359.4 +/- 22.3 nM)
- ChEMBL_2330608 Negative allosteric modulator activity at human P-gp Q1193T mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 523.6 +/- 33.7 nM)
- ChEMBL_2330609 Negative allosteric modulator activity at human P-gp Q1193N mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 560.2 +/- 43.9 nM)
- ChEMBL_2330610 Negative allosteric modulator activity at human P-gp G1114V mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 3.4 +/- 0.5 nM)
- ChEMBL_2330611 Negative allosteric modulator activity at human P-gp G1114I mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 10.2 +/- 2.4 nM)
- ChEMBL_2330612 Negative allosteric modulator activity at human P-gp G1114L mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 4.5 +/- 1.1 nM)
- ChEMBL_2330613 Negative allosteric modulator activity at human P-gp I1115W mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 7.8 +/- 0.9 nM)
- ChEMBL_2330614 Negative allosteric modulator activity at human P-gp I1115Y mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 6.9 +/- 1.2 nM)
- ChEMBL_2330615 Negative allosteric modulator activity at human P-gp I1115K mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 6.2 +/- 2 nM)
- ChEMBL_2330616 Negative allosteric modulator activity at human P-gp I1115E mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 7.4 +/- 0.8 nM)
- ChEMBL_2330617 Negative allosteric modulator activity at human P-gp I1115N mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 10.2 +/- 1.6 nM)
- ChEMBL_2330618 Negative allosteric modulator activity at human P-gp I1115M mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 9.8 +/- 0.9 nM)
- ChEMBL_2330619 Negative allosteric modulator activity at human P-gp I1115F mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 663.1 +/- 10.2 nM)
- ChEMBL_2330620 Negative allosteric modulator activity at human P-gp I1115L mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 472 +/- 15.4 nM)
- ChEMBL_2330621 Negative allosteric modulator activity at human P-gp T1226W mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 363.5 +/- 24.9 nM)
- ChEMBL_2330622 Negative allosteric modulator activity at human P-gp T1226Y mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 449.3 +/- 31.2 nM)
- ChEMBL_2330623 Negative allosteric modulator activity at human P-gp T1226F mutant expressed in HEK293FT cells assessed as reversal of P-gp mediated PTX resistance by measuring paclitaxel IC50 incubated for 5 days by MTS assay (Rvb = 409.9 +/- 19.7 nM)
- Inhibition Assay Six test compound concentrations (0.1, 0.25, 1, 2.5, 10, 25 μM in DMSO; final DMSO concentration=0.3%) are incubated with human liver microsomes (0.25 mg/mL) and NADPH (1 mM) in the presence of the probe substrate paclitaxel (7.5 μM) for 30 min at 37° C. The selective CYP2C8 inhibitor, montelukast, is screened alongside the test compounds as a positive control.
- Inhibition Assay Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at their previously determined Km values (CYP2C8: paclitaxel, 4 μM; CYP2C9: diclofenac, 5 μM; CYP3A4: midazolam, 0.5 μM). Incubations contained 0.1 mg/mL human liver microsomes, 3 mM MgCl2, probe substrate and various concentrations of inhibitor (12-point IC50 curve) in 100 mM potassium phosphate buffer (pH 7.4). Concentrations of organic solvents were kept to <1% (v/v). All incubations were pre-incubated at 37° C. for 5 minutes prior to addition of 1 mM NADPH (final concentration). Incubations were stopped after 5 (CYP3A4) or 15 minutes (CYP2C8 and CYP2C9) with one volume (v/v) of ice-cold acetonitrile containing 0.1 μM tolbutamide as an internal standard. All samples were vortexed and centrifuged prior to LC-MS/MS analysis.
- ADP Hunter Plus Assay Compounds were first tested in triplicates at 100 uM; hit compounds were further tested with a 10-point twofold serial dilution to confirm their activity and determine their IC50 values. S-Trityl-L-cysteine (STLC), a selective Eg5 inhibitor(J. Biol. Chem. 2006, 281:17559-17569; Mol. Cancer Ther. 2004, 3:1079-1090), was used as the control compound. Specifically, 20 uL of 15 mM PIPES (pH 7.0) containing 1 mM MgCl2, 50 nM MT, 20 uM paclitaxel, 200 uM ATP, 5% DMSO, 60 nM Eg5 proteins, and 1:2 serial dilutions of each individual compound starting from 1000 uM were added to each well of a 96-well plate. The plate was incubated at room temperature for 0.5 h, and then, the ADP Hunter Plus reagents were added. The plate was further incubated for 0.5 h and then read for fluorescence (ex.530/em.590) on Synergy 4 (BioTek, Winooski, VT, USA).
- Inhibitory Activities Against HSET-HSET ADP-Glo Assay Reagents: (+4° C. Storage)Paclitaxel Prod.No.TXD.01 2 mM in DMSO (from Universal Biologicals Cambridge).Tubulin Protein (Pre-formed Microtubules): Bovine Brain Prod.No.MT001-XL Lot.025 10 mg/mL (from Universal Biologicals Cambridge).Reagents: (−80° C. Storage)MT001-XL—Tubulin Protein (Pre-formed Microtubules): Bovine Brain—reconstituted in buffer—15 mM PIPES pH7, 1 mM MgCl2, 20 μM paclitaxel (aliquots at 10 mg/mL) from Universal Biologicals Cambridge.HSET full length protein—Current batch is FL HSET Prep1 (SEQ-000096_002-01_01)Buffer is 20 mM Hepes pH 7.5, 200 mM NaCl, 2 mM TCEP, 5% glycerol (5 μL aliquots at 10.2 μM)Reagents: (−20° C. Storage)ADP-Glo Kinase Assay kit (Promega Prod.No.V9102) 10,000 assay pointsADP-Glo reagent (50 mL), Kinase Detection Reagent (100 mL), and Ultrapure ATP (10 mM) aliquotedBuffer Stocks (filtered and stored at rt for up to 1 month)HEPES acid, 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid MW:238, 238.3 mg/mL=1 M7149 mg/30 mL=1M (pH to 6.8 with 5 M NaOH)PIPES, 1,4-Piperazinediethanesulfonic acid MW: 302.4, 30.24 mg/mL=100 mM; 907.2 mg/30 mL=100 mM (pH to 7 with 5 M NaOH, white cloudy suspension until the pH changes)EGTA, Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid MW: 380.35, 38.035 mg/mL=100 mM1901.75 mg/50 mL=100 mM (pH to ˜7 with 5 M NaOH)—takes a long time to get into solution and for pH to stabilizeTriton-X-100, MW: 625, 62.5 mg/mL=100 mM (6.25% w/v) (viscous, pipette Triton X-100 with a Gilson using a trimmed pipette tip)ECHO ProtocolCreate an ECHO intermediate plate by adding 24.5 μL DMSO to columns 1 & 2, and 40 μL DMSO to columns 23 & 24 of an ECHO 384PP plateAdd 100 nL of compound/DMSO per well in 384-well Proxi-Plate Plus (white) —Perkin Elmer Cat #6008289 using ECHO dose response protocol 100 nL normal to Assay buffer (Keep on ice): HEPES pH 6.8, MgCl2, EGTA, Triton X-100, DTT, HPLC H2O Microtubule Working Solution (3.2):1 ml PM buffer: PIPES pH 7.0, MgCl2, HPLC H2O, Paclitaxel, mix well, store at rtThen add 26.4 μL of 10 mg/ml microtubules to 724 μL of the above PM buffer to make 750 μL of microtubule working solution @350 μg/mL microtubules (store at rt)Stock HSET enzyme solution (3.1)—keep on iceAdd 1.33 μL 10.2 μM HSET Prepi (SEQ-000096_002-01_01) to 1358 μL assay buffer to make a 10 nM stock for a 5 nM final assay concentration (1/1020 dilution).HSET/Microtubule working solution (3.3)Mix solutions 3.1 and 3.2 in the ratio of 2.5:1 (1214.3 μL 3.1+485.7 μL 3.2) keep at rt for 15 min3.2 is diluted 3.5-fold, 3.1 is diluted 1.4-fold BLANK solution is 357 μL 2XAB+143 μL microtubule working solution 3.2. (Same proportions as HSET/Microtubule working solution (3.3))ATP Working solution is 1 μL 10 mM UltraPure ATP (Promega kit)+999 μL ddH20 gives 10 μM ATP for a 3 μM final assay concentration, stored on ice.Assay procedure in PROXIPLATE 384 PLUS WHITE (Perkin Elmer) plates (Remove 2.4 mL ADP Glo and 4.5 mL Kinase detection reagent from freezer to warm up to rt)Add 3.5 μL BLANK solution to assay plate (column 12)Add 3.5 μL HSET/Microtubule solution (3.3) (columns 1-11 & 13-24)Centrifuge at 1000 rpm for 1 min(pre-incubate enzyme and compound for 10 min)Add 1.5 μL of 10 μM ATP to start reaction gives a final [HSET] of 5 nM, [ATP] of 3 μM and [microtubule] of 70 μg/mL, centrifuge at 1000 rpm for 1 min and incubated at rt for 80 min.After the 80-minute incubation, stop reaction by adding 5 μL ADP-Glo reagent to all wells. Centrifuge for 1 min at 1000 rpm, leave for 40 min at rt.In the dark/away from direct light, add 10 μL Kinase Detection Reagent (KDR) to all wells, Seal plate with a Topseal (Perkin Elmer Cat #6050185) and centrifuge as above, leave for 40 min at rt covered in foil.
- KIF18A Enzyme Assay KIF18A enzyme assay: The enzymatic activity of KIF18A after treatment with the compounds was measured by an assay of microtubule-stimulated ATPase activity. ADP generated from the ATPase reaction was measured in this assay. The compounds were serially diluted 2-fold in DMSO over a range of 22 concentration points. Recombinant human KIF18A (1-467His-tagged) protein was expressed using a baculovirus system. Concentrations of KIF18A protein, microtubules, and ATP in the reaction were optimized for a standardized homogenous enzyme assay using an ADP-Glo kinase/ATPase assay kit. A reaction buffer [(15 mM Tris, pH 7.5), 10 mM MgCl2, 0.01% PluronicF-68, 1 μM paclitaxel, and 30 μg/mL pig microtubules] was prepared. The compounds and KIF18A protein (30 nM) were added to the prepared reaction buffer, and the reaction mixture was incubated at room temperature for 15 min, followed by addition of ATP (Km, 75 μM). The resulting reaction mixture was incubated at room temperature for another 15 min. 5 μL of ADP-Glo reagent and 2.5 μL of the reaction mixture were mixed and the resulting mixture was incubated at room temperature for 40 min. 10 μL of ADP-Glo detection reagent was added and the mixture was incubated at room temperature for 40 min. Luminescence was measured using a microplate reader and compared with that of the DMSO group, and then the inhibition percentages and IC50 values of the compounds were calculated.
- Assay on Inhibition of KIF18A Enzymatic Activity The reaction system consisted of a test compound, the recombinant protein KIF18A (aa 1-467), ATP (Promega Inc), and an assay buffer. The test compound was prepared into a 0.5 mM stock solution using DMSO (Sigma Inc) and then serially diluted in DMSO. The assay buffer consisted of an aqueous solution of 15 mM Tris, 10 mM MgCl2 (Sigma Inc), 0.01% Pluronic F-68 (Life Technologies Inc), 1 μM paclitaxel (Cytoskeleton Inc), 30 μg/mL porcine tubulin (Cytoskeleton Inc), and 2% DMSO. The KIF18A protein (final concentration: 80 nM) and the compound at different concentrations (1 μL) were added to the prepared assay buffer (50 μL), and the mixture was incubated at room temperature for 15 min. Then, ATP (final concentration: 80 μM) was added to the reaction mixture, and the resulting mixture was incubated at room temperature for 3 h. After the reaction was completed, 5 μL of the ADP-Glo™ reagent and 2.5 μL of the reaction mixture were added to a 384-well plate (Grenier Inc). The resulting mixture was mixed homogeneously, then sealed by an aluminum foil sealing film, and incubated at room temperature in the dark for 40 min. Finally, 10 μL of the ADP-GloTM assay reagent was added to each reaction well, and the mixture was incubated at room temperature in the dark for 40 min. After all reactions were completed, the luminescence values of the wells were read on a microplate reader (Molecular Device_SpectraMax Id5), and the inhibition ratios were calculated.
 taxol CHEMBL428647 BDBM50001839 PACLITAXEL
taxol CHEMBL428647 BDBM50001839 PACLITAXEL BDBM573947 US11453697, Example 132 2-amino-9-[(5S,7R,8R,12aR,14R,15S,15aR)- 14-(6-amino-9H-purin-9-yl)-15-fluoro-2,10- dihydroxy-2,10-disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-oe (Diastereomer 3)
BDBM573947 US11453697, Example 132 2-amino-9-[(5S,7R,8R,12aR,14R,15S,15aR)- 14-(6-amino-9H-purin-9-yl)-15-fluoro-2,10- dihydroxy-2,10-disulfidooctahydro-12H-5,8- methanofuro[3,2-l][1,3,6,9,11,2,10] pentaoxadiphosphacyclotetradecin-7-yl]-1,9- dihydro-6H-purin-6-oe (Diastereomer 3)