Target (11)
Compound (30)
Article Title (131)
Assay (96)
Bonazzi, S; Connolly, M; Glass, DJ; Mihalic, M; Patterson, AW; Roggo, S; Shavlakadze, T Rapamycin derivatives US Patent US12091424 (2024) NOVEL RAPAMYCIN DERIVATIVES Wagner, R; Mollison, KW; Liu, L; Henry, CL; Rosenberg, TA; Bamaung, N; Tu, N; Wiedeman, PE; Or, Y; Luly, JR; Lane, BC; Trevillyan, J; Chen, YW; Fey, T; Hsieh, G; Marsh, K; Nuss, M; Jacobson, PB; Wilcox, D; Carlson, RP; Carter, GW; Djuric, SW Rapamycin analogs with reduced systemic exposure. Bioorg Med Chem Lett 15: 5340 -3 (2005) Gregory, MA; Kendrew, SG; Moss, SJ; Wilkinson, B Rapamycin analogues and their pharmaceutical use US Patent US9505773 (2016) Edwards, SR; Wandless, TJ The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain. J Biol Chem 282: 13395 -401 (2007) Dickman, DA; Ding, H; Li, Q; Nilius, AM; Balli, DJ; Ballaron, SJ; Trevillyan, JM; Smith, ML; Seif, LS; Kim, K; Sarthy, A; Goldman, RC; Plattner, JJ; Bennani, YL Antifungal rapamycin analogues with reduced immunosuppressive activity. Bioorg Med Chem Lett 10: 1405 -8 (2000) Abdel-Magid, AF Rapalogs Potential as Practical Alternatives to Rapamycin. ACS Med Chem Lett 10: 843 -845 (2019) Zhou, W; Wang, C; Liu, Z; Gou, S Hypoxia-Activated Prodrugs with Dual COX-2/CA Inhibitory Effects on Attenuating Cardiac Inflammation under Hypoxia. J Med Chem 65: 13436 -13451 (2022) Wu, Y; Li, Z; McDonough, MA; Schofield, CJ; Zhang, X Inhibition of the Oxygen-Sensing Asparaginyl Hydroxylase Factor Inhibiting Hypoxia-Inducible Factor: A Potential Hypoxia Response Modulating Strategy. J Med Chem 64: 7189 -7209 (2021) Kang, X; Long, W; Ma, C; Wang, Y; Cao, H; Wang, Y; Tan, F; Hu, Y Compounds as hypoxia mimetics, and compositions and uses thereof US Patent US8742138 (2014) Fu, J; Lou, Y; He, Y Indane derivatives as hypoxia inducible factor-2(α) inhibitors US Patent US12077506 (2024) Abdel-Magid, AF Inhibitors of Hypoxia-Inducible Factors as Treatment for Cancer. ACS Med Chem Lett 11: 1079 -1080 (2020) Xia, Y; Choi, HK; Lee, K Recent advances in hypoxia-inducible factor (HIF)-1 inhibitors. Eur J Med Chem 49: 24 -40 (2012) Mortensen, DS; Perrin-Ninkovic, SM; Shevlin, G; Zhao, J; Packard, G; Bahmanyar, S; Correa, M; Elsner, J; Harris, R; Lee, BG; Papa, P; Parnes, JS; Riggs, JR; Sapienza, J; Tehrani, L; Whitefield, B; Apuy, J; Bisonette, RR; Gamez, JC; Hickman, M; Khambatta, G; Leisten, J; Peng, SX; Richardson, SJ; Cathers, BE; Canan, SS; Moghaddam, MF; Raymon, HK; Worland, P; Narla, RK; Fultz, KE; Sankar, S Discovery of mammalian target of rapamycin (mTOR) kinase inhibitor CC-223. J Med Chem 58: 5323 -33 (2015) Scheuermann, TH; Stroud, D; Sleet, CE; Bayeh, L; Shokri, C; Wang, H; Caldwell, CG; Longgood, J; MacMillan, JB; Bruick, RK; Gardner, KH; Tambar, UK Isoform-Selective and Stereoselective Inhibition of Hypoxia Inducible Factor-2. J Med Chem 58: 5930 -41 (2015) Shalwitz, R; Gardner, JH Methods for increasing the stabilization of hypoxia inducible factor-α US Patent US9278930 (2016) Ng, D; Arend, MP; Flippin, LA Naphthyridine derivatives as inhibitors of Hypoxia inducible factor (HIF) hydroxylase US Patent US9695170 (2017) Ng, D; Arend, MP; Flippin, LA Naphthyridine derivatives as inhibitors of hypoxia inducible factor (HIF) hydroxylase US Patent US8921389 (2014) Shimizu, K; Maruyama, M; Yasui, Y; Minegishi, H; Ban, HS; Nakamura, H Boron-containing phenoxyacetanilide derivatives as hypoxia-inducible factor (HIF)-1alpha inhibitors. Bioorg Med Chem Lett 20: 1453 -6 (2010) Wong, WW; O'Brien-Gortner, SF; Anderson, RF; Wilson, WR; Hay, MP; Dickson, BD Hypoxia-activated prodrugs of phenolic olaparib analogues for tumour-selective chemosensitisation. RSC Med Chem 14: 1309 -1330 (2023) Shalwitz, R; Gardner, JH Methods for increasing the stabilization of hypoxia inducible factor-1 alpha US Patent US8778412 (2014) Mohammed, KA; Jadulco, RC; Bugni, TS; Harper, MK; Sturdy, M; Ireland, CM Strongylophorines: natural product inhibitors of hypoxia-inducible factor-1 transcriptional pathway. J Med Chem 51: 1402 -5 (2008) Tan, C; de Noronha, RG; Devi, NS; Jabbar, AA; Kaluz, S; Liu, Y; Mooring, SR; Nicolaou, KC; Wang, B; Van Meir, EG Sulfonamides as a new scaffold for hypoxia inducible factor pathway inhibitors. Bioorg Med Chem Lett 21: 5528 -32 (2011) Sedrani, R; Jones, LH; Jutzi-Eme, AM; Schuler, W; Cottens, S Cleavage of the cyclohexyl-subunit of rapamycin results in loss of immunosuppressive activity. Bioorg Med Chem Lett 9: 459 -62 (1999) Zask, A; Kaplan, J; Verheijen, JC; Richard, DJ; Curran, K; Brooijmans, N; Bennett, EM; Toral-Barza, L; Hollander, I; Ayral-Kaloustian, S; Yu, K Morpholine derivatives greatly enhance the selectivity of mammalian target of rapamycin (mTOR) inhibitors. J Med Chem 52: 7942 -5 (2009) Zhu, R; Seow, HA; Baumann, RP; Ishiguro, K; Penketh, PG; Shyam, K; Sartorelli, AC Design of a hypoxia-activated prodrug inhibitor of O6-alkylguanine-DNA alkyltransferase. Bioorg Med Chem Lett 22: 6242 -7 (2012) Chowdhury, R; Candela-Lena, JI; Chan, MC; Greenald, DJ; Yeoh, KK; Tian, YM; McDonough, MA; Tumber, A; Rose, NR; Conejo-Garcia, A; Demetriades, M; Mathavan, S; Kawamura, A; Lee, MK; van Eeden, F; Pugh, CW; Ratcliffe, PJ; Schofield, CJ Selective Small Molecule Probes for the Hypoxia Inducible Factor (HIF) Prolyl Hydroxylases. ACS Chem Biol 8: 1488 -96 (2013) Nowak, P; Cole, DC; Brooijmans, N; Bursavich, MG; Curran, KJ; Ellingboe, JW; Gibbons, JJ; Hollander, I; Hu, Y; Kaplan, J; Malwitz, DJ; Toral-Barza, L; Verheijen, JC; Zask, A; Zhang, WG; Yu, K Discovery of potent and selective inhibitors of the mammalian target of rapamycin (mTOR) kinase. J Med Chem 52: 7081 -9 (2009) Lee, K; Lee, JH; Boovanahalli, SK; Jin, Y; Lee, M; Jin, X; Kim, JH; Hong, YS; Lee, JJ (Aryloxyacetylamino)benzoic acid analogues: A new class of hypoxia-inducible factor-1 inhibitors. J Med Chem 50: 1675 -84 (2007) Dat, NT; Jin, X; Lee, JH; Lee, D; Hong, YS; Lee, K; Kim, YH; Lee, JJ Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1. J Nat Prod 70: 1093 -7 (2007) Li, Z; Su, K; Jiang, Z; Yu, Y; You, Q; Zhang, X Photoactivatable Prolyl Hydroxylase 2 Inhibitors for Stabilizing the Hypoxia-Inducible Factor with Light. J Med Chem 62: 7583 -7588 (2019) Li, Z; You, Q; Zhang, X Small-Molecule Modulators of the Hypoxia-Inducible Factor Pathway: Development and Therapeutic Applications. J Med Chem 62: 5725 -5749 (2019) Kwon, DY; Lee, HE; Weitzel, DH; Park, K; Lee, SH; Lee, CT; Stephenson, TN; Park, H; Fitzgerald, MC; Chi, JT; Mook, RA; Dewhirst, MW; Lee, YM; Hong, J Synthesis and Biological Evaluation of Manassantin Analogues for Hypoxia-Inducible Factor 1α Inhibition. J Med Chem 58: 7659 -71 (2015) Huang, W; Huang, R; Attene-Ramos, MS; Sakamuru, S; Englund, EE; Inglese, J; Austin, CP; Xia, M Synthesis and evaluation of quinazolin-4-ones as hypoxia-inducible factor-1a inhibitors. Bioorg Med Chem Lett 21: 5239 -43 (2011) Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. Park, H; Choe, H; Hong, S Virtual screening and biochemical evaluation to identify new inhibitors of mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 24: 835 -8 (2014) Mooring, SR; Jin, H; Devi, NS; Jabbar, AA; Kaluz, S; Liu, Y; Van Meir, EG; Wang, B Design and synthesis of novel small-molecule inhibitors of the hypoxia inducible factor pathway. J Med Chem 54: 8471 -89 (2011) Minegishi, H; Fukashiro, S; Ban, HS; Nakamura, H Discovery of Indenopyrazoles as a New Class of Hypoxia Inducible Factor (HIF)-1 Inhibitors. ACS Med Chem Lett 4: 297 -301 (2013) Fu, J; Lou, Y; He, Y Hypoxia inducible factor-2(alpha) inhibitors and their use in the treatment of diseases US Patent US11420936 (2022) McKee, TC; Rabe, D; Bokesch, HR; Grkovic, T; Whitson, EL; Diyabalanage, T; Van Wyk, AW; Marcum, SR; Gardella, RS; Gustafson, KR; Linehan, WM; McMahon, JB; Bottaro, DP Inhibition of hypoxia inducible factor-2 transcription: isolation of active modulators from marine sponges. J Nat Prod 75: 1632 -6 (2012) Ghedira, D; Voissière, A; Peyrode, C; Kraiem, J; Gerard, Y; Maubert, E; Vivier, M; Miot-Noirault, E; Chezal, JM; Farhat, F; Weber, V Structure-activity relationship study of hypoxia-activated prodrugs for proteoglycan-targeted chemotherapy in chondrosarcoma. Eur J Med Chem 158: 51 -67 (2018) Chittiboyina, AG; Kumar, GM; Carvalho, PB; Liu, Y; Zhou, YD; Nagle, DG; Avery, MA Total synthesis and absolute configuration of laurenditerpenol: a hypoxia inducible factor-1 activation inhibitor. J Med Chem 50: 6299 -302 (2007) Venkatesan, AM; Chen, Z; dos Santos, O; Dehnhardt, C; Santos, ED; Ayral-Kaloustian, S; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Yu, K; Chaudhary, I; Mansour, TS PKI-179: an orally efficacious dual phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor. Bioorg Med Chem Lett 20: 5869 -73 (2010) Li, J; Xi, W; Li, X; Sun, H; Li, Y Advances in inhibition of protein-protein interactions targeting hypoxia-inducible factor-1 for cancer therapy. Bioorg Med Chem 27: 1145 -1158 (2019) Said, HM; Hagemann, C; Carta, F; Katzer, A; Polat, B; Staab, A; Scozzafava, A; Anacker, J; Vince, GH; Flentje, M; Supuran, CT Hypoxia induced CA9 inhibitory targeting by two different sulfonamide derivatives including acetazolamide in human glioblastoma. Bioorg Med Chem 21: 3949 -57 (2013) Dat, NT; Jin, X; Lee, K; Hong, YS; Kim, YH; Lee, JJ Hypoxia-inducible factor-1 inhibitory benzofurans and chalcone-derived diels-alder adducts from Morus species. J Nat Prod 72: 39 -43 (2009) Rami, M; Dubois, L; Parvathaneni, NK; Alterio, V; van Kuijk, SJ; Monti, SM; Lambin, P; De Simone, G; Supuran, CT; Winum, JY Hypoxia-targeting carbonic anhydrase IX inhibitors by a new series of nitroimidazole-sulfonamides/sulfamides/sulfamates. J Med Chem 56: 8512 -20 (2013) Cheng, W; Yuan, Y; Qiu, N; Peng, P; Sheng, R; Hu, Y Identification of novel 4-anilinoquinazoline derivatives as potent EGFR inhibitors both under normoxia and hypoxia. Bioorg Med Chem 22: 6796 -805 (2015) Conejo-Garcia, A; McDonough, MA; Loenarz, C; McNeill, LA; Hewitson, KS; Ge, W; Liénard, BM; Schofield, CJ; Clifton, IJ Structural basis for binding of cyclic 2-oxoglutarate analogues to factor-inhibiting hypoxia-inducible factor. Bioorg Med Chem Lett 20: 6125 -8 (2010) Nagao, S; Yamane, Y; Funasaka, S; Tanaka, K; Miyazaki, K; Kotake, Y; Kamata, J; Watanabe-Miyano, S; Toyama, O; Ozawa, Y; Mizui, Y; Okamoto, K; Ito, D Synthesis and structure-activity relationships of novel, potent, orally active hypoxia-inducible factor-1 inhibitors. Bioorg Med Chem 22: 5513 -29 (2014) Boovanahalli, SK; Jin, X; Jin, Y; Kim, JH; Dat, NT; Hong, YS; Lee, JH; Jung, SH; Lee, K; Lee, JJ Synthesis of (aryloxyacetylamino)-isonicotinic/nicotinic acid analogues as potent hypoxia-inducible factor (HIF)-1alpha inhibitors. Bioorg Med Chem Lett 17: 6305 -10 (2007) Venkateswarlu, V; Pathania, AS; Aravinda Kumar, KA; Mahajan, P; Nargotra, A; Vishwakarma, RA; Malik, FA; Sawant, SD 4-(N-Phenyl-N'-substituted benzenesulfonyl)-6-(4-hydroxyphenyl)quinolines as inhibitors of mammalian target of rapamycin. Bioorg Med Chem 23: 4237 -47 (2015) Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem 56: 3090 -101 (2013) De Simone, G; Vitale, RM; Di Fiore, A; Pedone, C; Scozzafava, A; Montero, JL; Winum, JY; Supuran, CT Carbonic anhydrase inhibitors: Hypoxia-activatable sulfonamides incorporating disulfide bonds that target the tumor-associated isoform IX. J Med Chem 49: 5544 -51 (2006) Theriault, JR; Felts, AS; Bates, BS; Perez, JR; Palmer, M; Gilbert, SR; Dawson, ES; Engers, JL; Lindsley, CW; Emmitte, KA Discovery of a new molecular probe ML228: an activator of the hypoxia inducible factor (HIF) pathway. Bioorg Med Chem Lett 22: 76 -81 (2011) Khalil, OM; Kamal, AM; Bua, S; El Sayed Teba, H; Nissan, YM; Supuran, CT Pyrrolo and pyrrolopyrimidine sulfonamides act as cytotoxic agents in hypoxia via inhibition of transmembrane carbonic anhydrases. Eur J Med Chem 188: (2020) Zask, A; Verheijen, JC; Curran, K; Kaplan, J; Richard, DJ; Nowak, P; Malwitz, DJ; Brooijmans, N; Bard, J; Svenson, K; Lucas, J; Toral-Barza, L; Zhang, WG; Hollander, I; Gibbons, JJ; Abraham, RT; Ayral-Kaloustian, S; Mansour, TS; Yu, K ATP-Competitive Inhibitors of the Mammalian Target of Rapamycin: Design and Synthesis of Highly Potent and Selective Pyrazolopyrimidines J Med Chem 52: 5013 -6 (2009) Bonazzi, S; Goold, CP; Gray, A; Thomsen, NM; Nunez, J; Karki, RG; Gorde, A; Biag, JD; Malik, HA; Sun, Y; Liang, G; Lubicka, D; Salas, S; Labbe-Giguere, N; Keaney, EP; McTighe, S; Liu, S; Deng, L; Piizzi, G; Lombardo, F; Burdette, D; Dodart, JC; Wilson, CJ; Peukert, S; Curtis, D; Hamann, LG; Murphy, LO Discovery of a Brain-Penetrant ATP-Competitive Inhibitor of the Mechanistic Target of Rapamycin (mTOR) for CNS Disorders. J Med Chem 63: 1068 -1083 (2020) Ballou, LM; Selinger, ES; Choi, JY; Drueckhammer, DG; Lin, RZ Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl)pyrimido[2,1-alpha]isoquinolin-4-one. J Biol Chem 282: 24463 -70 (2007) Tao, Z; Barker, J; Shi, SD; Gehring, M; Sun, S Steady-state kinetic and inhibition studies of the mammalian target of rapamycin (mTOR) kinase domain and mTOR complexes. Biochemistry 49: 8488 -98 (2010) Velentza, AV; Wainwright, MS; Zasadzki, M; Mirzoeva, S; Schumacher, AM; Haiech, J; Focia, PJ; Egli, M; Watterson, DM An aminopyridazine-based inhibitor of a pro-apoptotic protein kinase attenuates hypoxia-ischemia induced acute brain injury. Bioorg Med Chem Lett 13: 3465 -70 (2003) Murray, JK; Balan, C; Allgeier, AM; Kasparian, A; Viswanadhan, V; Wilde, C; Allen, JR; Yoder, SC; Biddlecome, G; Hungate, RW; Miranda, LP Dipeptidyl-quinolone derivatives inhibit hypoxia inducible factor-1a prolyl hydroxylases-1, -2, and -3 with altered selectivity. J Comb Chem 12: 676 -86 (2010) Discovery of DS44470011: An oral hypoxia-inducible factor prolyl hydroxylase inhibitor for the treatment of renal anemia. Frohn, M; Viswanadhan, V; Pickrell, AJ; Golden, JE; Muller, KM; Bürli, RW; Biddlecome, G; Yoder, SC; Rogers, N; Dao, JH; Hungate, R; Allen, JR Structure-guided design of substituted aza-benzimidazoles as potent hypoxia inducible factor-1alpha prolyl hydroxylase-2 inhibitors. Bioorg Med Chem Lett 18: 5023 -6 (2008) Nakashima, H; Uto, Y; Nakata, E; Nagasawa, H; Ikkyu, K; Hiraoka, N; Nakashima, K; Sasaki, Y; Sugimoto, H; Shiro, Y; Hashimoto, T; Okamoto, Y; Asakawa, Y; Hori, H Synthesis and biological activity of 1-methyl-tryptophan-tirapazamine hybrids as hypoxia-targeting indoleamine 2,3-dioxygenase inhibitors. Bioorg Med Chem 16: 8661 -9 (2008) Naik, R; Won, M; Kim, BK; Xia, Y; Choi, HK; Jin, G; Jung, Y; Kim, HM; Lee, K Synthesis and structure-activity relationship of (E)-phenoxyacrylic amide derivatives as hypoxia-inducible factor (HIF) 1a inhibitors. J Med Chem 55: 10564 -71 (2012) D'Angelo, ND; Kim, TS; Andrews, K; Booker, SK; Caenepeel, S; Chen, K; D'Amico, D; Freeman, D; Jiang, J; Liu, L; McCarter, JD; San Miguel, T; Mullady, EL; Schrag, M; Subramanian, R; Tang, J; Wahl, RC; Wang, L; Whittington, DA; Wu, T; Xi, N; Xu, Y; Yakowec, P; Yang, K; Zalameda, LP; Zhang, N; Hughes, P; Norman, MH Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem 54: 1789 -811 (2011) Yoon, S; Kim, JH; Kim, SE; Kim, C; Tran, PT; Ann, J; Koh, Y; Jang, J; Kim, S; Moon, HS; Kim, WK; Lee, S; Lee, J; Kim, S; Lee, J Discovery of Leucyladenylate Sulfamates as Novel Leucyl-tRNA Synthetase (LRS)-Targeted Mammalian Target of Rapamycin Complex 1 (mTORC1) Inhibitors. J Med Chem 59: 10322 -10328 (2016) Cohen, F; Bergeron, P; Blackwood, E; Bowman, KK; Chen, H; Dipasquale, AG; Epler, JA; Koehler, MF; Lau, K; Lewis, C; Liu, L; Ly, CQ; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Robarge, KD; Sideris, S; Trinh, L; Truong, T; Wu, J; Zhao, X; Lyssikatos, JP Potent, selective, and orally bioavailable inhibitors of mammalian target of rapamycin (mTOR) kinase based on a quaternary substituted dihydrofuropyrimidine. J Med Chem 54: 3426 -35 (2011) Koehler, MF; Bergeron, P; Blackwood, E; Bowman, KK; Chen, YH; Deshmukh, G; Ding, X; Epler, J; Lau, K; Lee, L; Liu, L; Ly, C; Malek, S; Nonomiya, J; Oeh, J; Ortwine, DF; Sampath, D; Sideris, S; Trinh, L; Truong, T; Wu, J; Pei, Z; Lyssikatos, JP Potent, selective, and orally bioavailable inhibitors of the mammalian target of rapamycin kinase domain exhibiting single agent antiproliferative activity. J Med Chem 55: 10958 -71 (2012) Richard, DJ; Verheijen, JC; Yu, K; Zask, A Triazines incorporating (R)-3-methylmorpholine are potent inhibitors of the mammalian target of rapamycin (mTOR) with selectivity over PI3Kalpha. Bioorg Med Chem Lett 20: 2654 -7 (2010) Uno, M; Ban, HS; Nakamura, H 1-[4-(N-Benzylamino)phenyl]-3-phenylurea derivatives as a new class of hypoxia-inducible factor-1alpha inhibitors. Bioorg Med Chem Lett 19: 3166 -9 (2009) Dat, NT; Jin, X; Hong, YS; Lee, JJ An isoaurone and other constituents from Trichosanthes kirilowii seeds inhibit hypoxia-inducible factor-1 and nuclear factor-kappaB. J Nat Prod 73: 1167 -9 (2010) Kwon, HS; Kim, DR; Yang, EG; Park, YK; Ahn, HC; Min, SJ; Ahn, DR Inhibition of VEGF transcription through blockade of the hypoxia inducible factor-1α-p300 interaction by a small molecule. Bioorg Med Chem Lett 22: 5249 -52 (2012) Naik, R; Won, M; Ban, HS; Bhattarai, D; Xu, X; Eo, Y; Hong, YS; Singh, S; Choi, Y; Ahn, HC; Lee, K Synthesis and structure-activity relationship study of chemical probes as hypoxia induced factor-1a/malate dehydrogenase 2 inhibitors. J Med Chem 57: 9522 -38 (2014) Li, G; Shao, Y; Pan, Y; Li, Y; Wang, Y; Wang, L; Wang, X; Shao, K; Wang, S; Liu, N; Zhang, J; Zhao, W; Nakamura, H Total synthesis and biological evaluation of 7-hydroxyneolamellarin A as hypoxia-inducible factor-1α inhibitor for cancer therapy. Bioorg Med Chem Lett 50: (2021) Chen, Y; Yuan, X; Zhang, W; Tang, M; Zheng, L; Wang, F; Yan, W; Yang, S; Wei, Y; He, J; Chen, L Discovery of Novel Dual Histone Deacetylase and Mammalian Target of Rapamycin Target Inhibitors as a Promising Strategy for Cancer Therapy. J Med Chem 62: 1577 -1592 (2019) Yoon, S; Kim, JH; Koh, Y; Tran, PT; Ann, J; Yoon, I; Jang, J; Kim, WK; Lee, S; Lee, J; Kim, S; Lee, J Discovery of simplified leucyladenylate sulfamates as novel leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. Bioorg Med Chem 25: 4145 -4152 (2017) Bursavich, MG; Brooijmans, N; Feldberg, L; Hollander, I; Kim, S; Lombardi, S; Park, K; Mallon, R; Gilbert, AM Novel benzofuran-3-one indole inhibitors of PI3 kinase-alpha and the mammalian target of rapamycin: hit to lead studies. Bioorg Med Chem Lett 20: 2586 -90 (2010) Mortensen, DS; Perrin-Ninkovic, SM; Shevlin, G; Elsner, J; Zhao, J; Whitefield, B; Tehrani, L; Sapienza, J; Riggs, JR; Parnes, JS; Papa, P; Packard, G; Lee, BG; Harris, R; Correa, M; Bahmanyar, S; Richardson, SJ; Peng, SX; Leisten, J; Khambatta, G; Hickman, M; Gamez, JC; Bisonette, RR; Apuy, J; Cathers, BE; Canan, SS; Moghaddam, MF; Raymon, HK; Worland, P; Narla, RK; Fultz, KE; Sankar, S Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. J Med Chem 58: 5599 -608 (2015) Wei, H; Duan, Y; Gou, W; Cui, J; Ning, H; Li, D; Qin, Y; Liu, Q; Li, Y Design, synthesis and biological evaluation of novel 4-anilinoquinazoline derivatives as hypoxia-selective EGFR and VEGFR-2 dual inhibitors. Eur J Med Chem 181: (2019) Fuse, S; Ohuchi, T; Asawa, Y; Sato, S; Nakamura, H Development of 1-aryl-3-furanyl/thienyl-imidazopyridine templates for inhibitors against hypoxia inducible factor (HIF)-1 transcriptional activity. Bioorg Med Chem Lett 26: 5887 -5890 (2016) Liu, M; Liang, Y; Zhu, Z; Wang, J; Cheng, X; Cheng, J; Xu, B; Li, R; Liu, X; Wang, Y Discovery of Novel Aryl Carboxamide Derivatives as Hypoxia-Inducible Factor 1α Signaling Inhibitors with Potent Activities of Anticancer Metastasis. J Med Chem 62: 9299 -9314 (2019) Recent progress in the development of hypoxia-inducible factor 2α (HIF-2α) modulators: Inhibitors, agonists, and degraders (2009-2024). Zhou, YD; Kim, YP; Mohammed, KA; Jones, DK; Muhammad, I; Dunbar, DC; Nagle, DG Terpenoid tetrahydroisoquinoline alkaloids emetine, klugine, and isocephaeline inhibit the activation of hypoxia-inducible factor-1 in breast tumor cells. J Nat Prod 68: 947 -50 (2005) Curran, KJ; Verheijen, JC; Kaplan, J; Richard, DJ; Toral-Barza, L; Hollander, I; Lucas, J; Ayral-Kaloustian, S; Yu, K; Zask, A Pyrazolopyrimidines as highly potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 1-substituent. Bioorg Med Chem Lett 20: 1440 -4 (2010) Yasuda, Y; Arakawa, T; Nawata, Y; Shimada, S; Oishi, S; Fujii, N; Nishimura, S; Hattori, A; Kakeya, H Design, synthesis, and structure-activity relationships of 1-ethylpyrazole-3-carboxamide compounds as novel hypoxia-inducible factor (HIF)-1 inhibitors. Bioorg Med Chem 23: 1776 -87 (2015) Liu, P; Wang, L; DuBois, BG; Colandrea, VJ; Liu, R; Cai, J; Du, X; Quan, W; Morris, W; Bai, J; Bishwokarma, B; Cheng, M; Piesvaux, J; Ray, K; Alpert, C; Chiu, CS; Zielstorff, M; Metzger, JM; Yang, L; Leung, D; Alleyne, C; Vincent, SH; Pucci, V; Li, X; Crespo, A; Stickens, D; Hale, JJ; Ujjainwalla, F; Sinz, CJ Discovery of Orally Bioavailable and Liver-Targeted Hypoxia-Inducible Factor Prolyl Hydroxylase (HIF-PHD) Inhibitors for the Treatment of Anemia. ACS Med Chem Lett 9: 1193 -1198 (2018) Rabinowitz, MH Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: tricking the body into mounting orchestrated survival and repair responses. J Med Chem 56: 9369 -402 (2014) Cai, XF; Jin, X; Lee, D; Yang, YT; Lee, K; Hong, YS; Lee, JH; Lee, JJ Phenanthroquinolizidine alkaloids from the roots of Boehmeria pannosa potently inhibit hypoxia-inducible factor-1 in AGS human gastric cancer cells. J Nat Prod 69: 1095 -7 (2006) Tsou, HR; MacEwan, G; Birnberg, G; Zhang, N; Brooijmans, N; Toral-Barza, L; Hollander, I; Ayral-Kaloustian, S; Yu, K 4-Substituted-7-azaindoles bearing a ureidobenzofuranone moiety as potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 2259 -63 (2010) Takeuchi, CS; Kim, BG; Blazey, CM; Ma, S; Johnson, HW; Anand, NK; Arcalas, A; Baik, TG; Buhr, CA; Cannoy, J; Epshteyn, S; Joshi, A; Lara, K; Lee, MS; Wang, L; Leahy, JW; Nuss, JM; Aay, N; Aoyama, R; Foster, P; Lee, J; Lehoux, I; Munagala, N; Plonowski, A; Rajan, S; Woolfrey, J; Yamaguchi, K; Lamb, P; Miller, N Discovery of a novel class of highly potent, selective, ATP-competitive, and orally bioavailable inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem 56: 2218 -34 (2013) Dehnhardt, CM; Venkatesan, AM; Delos Santos, E; Chen, Z; Santos, O; Ayral-Kaloustian, S; Brooijmans, N; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Chaudhary, I; Yu, K; Gibbons, J; Abraham, R; Mansour, TS Lead optimization of N-3-substituted 7-morpholinotriazolopyrimidines as dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors: discovery of PKI-402. J Med Chem 53: 798 -810 (2010) Castro-Falcón, G; Seiler, GS; Demir, Ö; Rathinaswamy, MK; Hamelin, D; Hoffmann, RM; Makowski, SL; Letzel, AC; Field, SJ; Burke, JE; Amaro, RE; Hughes, CC Neolymphostin A Is a Covalent Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitor That Employs an Unusual Electrophilic Vinylogous Ester. J Med Chem 61: 10463 -10472 (2018) Stec, MM; Andrews, KL; Bo, Y; Caenepeel, S; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Whittington, DA; Wang, L; Wu, T; Zalameda, LP; Zhang, N; Hughes, PE; Norman, MH The imidazo[1,2-a]pyridine ring system as a scaffold for potent dual phosphoinositide-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitors. Bioorg Med Chem Lett 25: 4136 -42 (2015) Fuse, S; Suzuki, K; Kuchimaru, T; Kadonosono, T; Ueda, H; Sato, S; Kizaka-Kondoh, S; Nakamura, H Design, synthesis, and evaluation of indeno[2,1-c]pyrazolones for use as inhibitors against hypoxia-inducible factor (HIF)-1 transcriptional activity. Bioorg Med Chem 28: (2020) Wu, CZ; Hong, SS; Cai, XF; Dat, NT; Nan, JX; Hwang, BY; Lee, JJ; Lee, D Hypoxia-inducible factor-1 and nuclear factor-kappaB inhibitory meroterpene analogues of bakuchiol, a constituent of the seeds of Psoralea corylifolia. Bioorg Med Chem Lett 18: 2619 -23 (2008) Lee, S; Kwon, OS; Lee, CS; Won, M; Ban, HS; Ra, CS Synthesis and biological evaluation of kresoxim-methyl analogues as novel inhibitors of hypoxia-inducible factor (HIF)-1 accumulation in cancer cells. Bioorg Med Chem Lett 27: 3026 -3029 (2017) Pan, Z; Chen, Y; Pang, H; Wang, X; Zhang, Y; Xie, X; He, G Design, synthesis, and biological evaluation of novel dual inhibitors of heat shock protein 90/mammalian target of rapamycin (Hsp90/mTOR) against bladder cancer cells. Eur J Med Chem 242: (2022) Nishimura, N; Siegmund, A; Liu, L; Yang, K; Bryan, MC; Andrews, KL; Bo, Y; Booker, SK; Caenepeel, S; Freeman, D; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Wang, L; Whittington, DA; Zalameda, L; Zhang, N; Hughes, PE; Norman, MH Phospshoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors: discovery and structure-activity relationships of a series of quinoline and quinoxaline derivatives. J Med Chem 54: 4735 -51 (2011) Stec, MM; Andrews, KL; Booker, SK; Caenepeel, S; Freeman, DJ; Jiang, J; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Wang, L; Yang, K; Zalameda, LP; Zhang, N; Hughes, PE; Norman, MH Structure-activity relationships of phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors: investigations of various 6,5-heterocycles to improve metabolic stability. J Med Chem 54: 5174 -84 (2011) Sakai, M; Takahashi, N; Ikeda, H; Furutani, Y; Higuchi, S; Suzuki, T; Dohmae, N; Kobayashi, S; Harada, H; Kojima, S; Matsuura, T; Hattori, A; Kakeya, H Design, synthesis, and target identification of new hypoxia-inducible factor 1 (HIF-1) inhibitors containing 1-alkyl-1H-pyrazole-3-carboxamide moiety. Bioorg Med Chem 46: (2021) Liu, Y; Liu, R; Mao, SC; Morgan, JB; Jekabsons, MB; Zhou, YD; Nagle, DG Molecular-targeted antitumor agents. 19. Furospongolide from a marine Lendenfeldia sp. sponge inhibits hypoxia-inducible factor-1 activation in breast tumor cells. J Nat Prod 71: 1854 -60 (2008) An, H; Lee, S; Lee, JM; Jo, DH; Kim, J; Jeong, YS; Heo, MJ; Cho, CS; Choi, H; Seo, JH; Hwang, S; Lim, J; Kim, T; Jun, HO; Sim, J; Lim, C; Hur, J; Ahn, J; Kim, HS; Seo, SY; Na, Y; Kim, SH; Lee, J; Lee, J; Chung, SJ; Kim, YM; Kim, KW; Kim, SG; Kim, JH; Suh, YG Novel Hypoxia-Inducible Factor 1α (HIF-1α) Inhibitors for Angiogenesis-Related Ocular Diseases: Discovery of a Novel Scaffold via Ring-Truncation Strategy. J Med Chem 61: 9266 -9286 (2018) Boccellino, M; Donniacuo, M; Bruno, F; Rinaldi, B; Quagliuolo, L; Ambruosi, M; Pace, S; De Rosa, M; Olgaç, A; Banoglu, E; Alessio, N; Massa, A; Kahn, H; Werz, O; Fiorentino, A; Filosa, R Protective effect of piceatannol and bioactive stilbene derivatives against hypoxia-induced toxicity in H9c2 cardiomyocytes and structural elucidation as 5-LOX inhibitors. Eur J Med Chem 180: 637 -647 (2019) Wendt, B; Mulbaier, M; Wawro, S; Schultes, C; Alonso, J; Janssen, B; Lewis, J Toluidinesulfonamide hypoxia-induced factor 1 inhibitors: alleviating drug-drug interactions through use of PubChem data and comparative molecular field analysis guided synthesis. J Med Chem 54: 3982 -6 (2011) Nocentini, A; Trallori, E; Singh, S; Lomelino, CL; Bartolucci, G; Di Cesare Mannelli, L; Ghelardini, C; McKenna, R; Gratteri, P; Supuran, CT 4-Hydroxy-3-nitro-5-ureido-benzenesulfonamides Selectively Target the Tumor-Associated Carbonic Anhydrase Isoforms IX and XII Showing Hypoxia-Enhanced Antiproliferative Profiles. J Med Chem 61: 10860 -10874 (2018) Discovery of novel diaryl substituted isoquinolin-1(2H)-one derivatives as hypoxia-inducible factor-1 signaling inhibitors for the treatment of rheumatoid arthritis. Jia, T; Miao, R; Zhang, J; Zhu, H; Zhang, C; Zeng, L; Zhao, Y; Cheng, W; Shao, J Discovery of novel hypoxia-activated, nitroimidazole constructed multi-target kinase inhibitors on the basis of AZD9291 for the treatment of human lung cancer. Bioorg Med Chem 91: (2023) Hodges, TW; Hossain, CF; Kim, YP; Zhou, YD; Nagle, DG Molecular-targeted antitumor agents: the Saururus cernuus dineolignans manassantin B and 4-O-demethylmanassantin B are potent inhibitors of hypoxia-activated HIF-1. J Nat Prod 67: 767 -71 (2004) Zwergel, C; Aventaggiato, M; Garbo, S; Di Bello, E; Fassari, B; Noce, B; Castiello, C; Lambona, C; Barreca, F; Rotili, D; Fioravanti, R; Schmalz, T; Weyand, M; Niedermeier, A; Tripodi, M; Colotti, G; Steegborn, C; Battistelli, C; Tafani, M; Valente, S; Mai, A Novel 1,4-Dihydropyridines as Specific Binders and Activators of SIRT3 Impair Cell Viability and Clonogenicity and Downregulate Hypoxia-Induced Targets in Cancer Cells. J Med Chem 66: 9622 -9641 (2023) Luengo, JI; Yamashita, DS; Dunnington, D; Beck, AK; Rozamus, LW; Yen, HK; Bossard, MJ; Levy, MA; Hand, A; Newman-Tarr, T Structure-activity studies of rapamycin analogs: evidence that the C-7 methoxy group is part of the effector domain and positioned at the FKBP12-FRAP interface. Chem Biol 2: 471 -81 (1995) Cheng, W; Zhu, S; Ma, X; Qiu, N; Peng, P; Sheng, R; Hu, Y Design, synthesis and biological evaluation of 6-(nitroimidazole-1H-alkyloxyl)-4-anilinoquinazolines as efficient EGFR inhibitors exerting cytotoxic effects both under normoxia and hypoxia. Eur J Med Chem 89: 826 -34 (2014) Sutherlin, DP; Bao, L; Berry, M; Castanedo, G; Chuckowree, I; Dotson, J; Folks, A; Friedman, L; Goldsmith, R; Gunzner, J; Heffron, T; Lesnick, J; Lewis, C; Mathieu, S; Murray, J; Nonomiya, J; Pang, J; Pegg, N; Prior, WW; Rouge, L; Salphati, L; Sampath, D; Tian, Q; Tsui, V; Wan, NC; Wang, S; Wei, B; Wiesmann, C; Wu, P; Zhu, BY; Olivero, A Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem 54: 7579 -87 (2011) Vachal, P; Miao, S; Pierce, JM; Guiadeen, D; Colandrea, VJ; Wyvratt, MJ; Salowe, SP; Sonatore, LM; Milligan, JA; Hajdu, R; Gollapudi, A; Keohane, CA; Lingham, RB; Mandala, SM; DeMartino, JA; Tong, X; Wolff, M; Steinhuebel, D; Kieczykowski, GR; Fleitz, FJ; Chapman, K; Athanasopoulos, J; Adam, G; Akyuz, CD; Jena, DK; Lusen, JW; Meng, J; Stein, BD; Xia, L; Sherer, EC; Hale, JJ 1,3,8-Triazaspiro[4.5]decane-2,4-diones as efficacious pan-inhibitors of hypoxia-inducible factor prolyl hydroxylase 1-3 (HIF PHD1-3) for the treatment of anemia. J Med Chem 55: 2945 -59 (2012) Venkatesan, AM; Dehnhardt, CM; Delos Santos, E; Chen, Z; Dos Santos, O; Ayral-Kaloustian, S; Khafizova, G; Brooijmans, N; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Yu, K; Gibbons, J; Abraham, RT; Chaudhary, I; Mansour, TS Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5'-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem 53: 2636 -45 (2010) Vu, LP; Diehl, CJ; Casement, R; Bond, AG; Steinebach, C; Strašek, N; Bricelj, A; Perdih, A; Schnakenburg, G; Sosič, I; Ciulli, A; Gütschow, M Expanding the Structural Diversity at the Phenylene Core of Ligands for the von Hippel-Lindau E3 Ubiquitin Ligase: Development of Highly Potent Hypoxia-Inducible Factor-1α Stabilizers. J Med Chem 66: 12776 -12811 (2023) Yu, Y; Yang, F; Yu, Q; Liu, S; Wu, C; Su, K; Yang, L; Bao, X; Li, Z; Li, X; Zhang, X Discovery of a Potent and Orally Bioavailable Hypoxia-Inducible Factor 2α (HIF-2α) Agonist and Its Synergistic Therapy with Prolyl Hydroxylase Inhibitors for the Treatment of Renal Anemia. J Med Chem 64: 17384 -17402 (2021) Dai, J; Liu, Y; Jia, H; Zhou, YD; Nagle, DG Benzochromenones from the marine crinoid Comantheria rotula inhibit hypoxia-inducible factor-1 (HIF-1) in cell-based reporter assays and differentially suppress the growth of certain tumor cell lines. J Nat Prod 70: 1462 -6 (2007) Dai, J; Fishback, JA; Zhou, YD; Nagle, DG Sodwanone and yardenone triterpenes from a South African species of the marine sponge Axinella inhibit hypoxia-inducible factor-1 (HIF-1) activation in both breast and prostate tumor cells. J Nat Prod 69: 1715 -20 (2006) Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem 52: 8010 -24 (2009) Tsou, HR; MacEwan, G; Birnberg, G; Grosu, G; Bursavich, MG; Bard, J; Brooijmans, N; Toral-Barza, L; Hollander, I; Mansour, TS; Ayral-Kaloustian, S; Yu, K Discovery and optimization of 2-(4-substituted-pyrrolo[2,3-b]pyridin-3-yl)methylene-4-hydroxybenzofuran-3(2H)-ones as potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 2321 -5 (2010) Mun, J; Jabbar, AA; Devi, NS; Yin, S; Wang, Y; Tan, C; Culver, D; Snyder, JP; Van Meir, EG; Goodman, MM Design and in vitro activities of N-alkyl-N-[(8-R-2,2-dimethyl-2H-chromen-6-yl)methyl]heteroarylsulfonamides, novel, small-molecule hypoxia inducible factor-1 pathway inhibitors and anticancer agents. J Med Chem 55: 6738 -50 (2012) Kaplan, J; Verheijen, JC; Brooijmans, N; Toral-Barza, L; Hollander, I; Yu, K; Zask, A Discovery of 3,6-dihydro-2H-pyran as a morpholine replacement in 6-aryl-1H-pyrazolo[3,4-d]pyrimidines and 2-arylthieno[3,2-d]pyrimidines: ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 640 -3 (2010) Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Ahmed, T; Sabatini, DM; Gray, NS Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem 54: 1473 -80 (2011) Wu, Y; Jiang, Z; Li, Z; Gu, J; You, Q; Zhang, X Click Chemistry-Based Discovery of [3-Hydroxy-5-(1 H-1,2,3-triazol-4-yl)picolinoyl]glycines as Orally Active Hypoxia-Inducing Factor Prolyl Hydroxylase Inhibitors with Favorable Safety Profiles for the Treatment of Anemia. J Med Chem 61: 5332 -5349 (2018) Galdeano, C; Gadd, MS; Soares, P; Scaffidi, S; Van Molle, I; Birced, I; Hewitt, S; Dias, DM; Ciulli, A Structure-guided design and optimization of small molecules targeting the protein-protein interaction between the von Hippel-Lindau (VHL) E3 ubiquitin ligase and the hypoxia inducible factor (HIF) alpha subunit with in vitro nanomolar affinities. J Med Chem 57: 8657 -63 (2014) Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53: 7146 -55 (2010) Debenham, JS; Madsen-Duggan, C; Clements, MJ; Walsh, TF; Kuethe, JT; Reibarkh, M; Salowe, SP; Sonatore, LM; Hajdu, R; Milligan, JA; Visco, DM; Zhou, D; Lingham, RB; Stickens, D; DeMartino, JA; Tong, X; Wolff, M; Pang, J; Miller, RR; Sherer, EC; Hale, JJ Discovery of N-[Bis(4-methoxyphenyl)methyl]-4-hydroxy-2-(pyridazin-3-yl)pyrimidine-5-carboxamide (MK-8617), an Orally Active Pan-Inhibitor of Hypoxia-Inducible Factor Prolyl Hydroxylase 1-3 (HIF PHD1-3) for the Treatment of Anemia. J Med Chem 59: 11039 -11049 (2016) Mun, J; Jabbar, AA; Devi, NS; Liu, Y; Van Meir, EG; Goodman, MM Structure-activity relationship of 2,2-dimethyl-2H-chromene based arylsulfonamide analogs of 3,4-dimethoxy-N-[(2,2-dimethyl-2H-chromen-6-yl)methyl]-N-phenylbenzenesulfonamide, a novel small molecule hypoxia inducible factor-1 (HIF-1) pathway inhibitor and anti-cancer agent. Bioorg Med Chem 20: 4590 -7 (2012) Wehn, PM; Rizzi, JP; Dixon, DD; Grina, JA; Schlachter, ST; Wang, B; Xu, R; Yang, H; Du, X; Han, G; Wang, K; Cao, Z; Cheng, T; Czerwinski, RM; Goggin, BS; Huang, H; Halfmann, MM; Maddie, MA; Morton, EL; Olive, SR; Tan, H; Xie, S; Wong, T; Josey, JA; Wallace, EM Design and Activity of Specific Hypoxia-Inducible Factor-2α (HIF-2α) Inhibitors for the Treatment of Clear Cell Renal Cell Carcinoma: Discovery of Clinical Candidate ( S)-3-((2,2-Difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1 H-inden-4-yl)oxy)-5-fluorobenzonitrile (PT2385). J Med Chem 61: 9691 -9721 (2018)
FKBP12 Assay FKBP12 assay using rapamycin analogs. ChEMBL_305479 (CHEMBL830270) Inhibition of Mammalian target of Rapamycin mTOR ChEMBL_2430724 Inhibition of hypoxia induced VEGFR-2 (unknown origin) ChEMBL_750098 (CHEMBL1787515) Inhibition of hypoxia-induced HIF1alpha transcriptional activity in HEK293 cells incubated for 16 hrs by hypoxia response element-driven luciferase reporter gene assay ChEMBL_481746 (CHEMBL1002847) Inhibition of hypoxia-induced HIF1 activation in human U251 cells ChEMBL_1989567 (CHEMBL4623302) Inhibition of Vps34 in human MCF7-LC3 cells assessed as rapamycin-induced autophagy ChEMBL_321108 (CHEMBL882874) Effective concentration to inhibit production of hypoxia inducible factor 1-alpha protein ChEMBL_475987 (CHEMBL931441) Inhibition of hypoxia-induced HIF1 activation in human AGS cells by reporter gene assay ChEMBL_527926 (CHEMBL975114) Inhibition of hypoxia-induced HIF1 activation in human T47D cells by reporter gene assay ChEMBL_527928 (CHEMBL975116) Inhibition of hypoxia-induced HIF1 activation in human PC3 cells by reporter gene assay ChEMBL_767399 (CHEMBL1825511) Inhibition of hypoxia-induced HIF1 activation in human LN229 cells expressing HRE-AP reporter gene preincubated for 1 hr under normoxia condition followed by 24 hrs incubation under hypoxia condition by HRE-luciferase reporter assay ChEMBL_1294789 (CHEMBL3128876) Inhibition of Stat3 phosphorylation in human OVCAR3 cells by Western blotting analysis in hypoxia condition ChEMBL_1294791 (CHEMBL3128878) Inhibition of Stat3 phosphorylation in human PANC1 cells by Western blotting analysis in hypoxia condition ChEMBL_550371 (CHEMBL999925) Inhibition of hypoxia-induced HIF1 activation in human HeLa cells by luciferase reporter gene assay ChEMBL_1294796 (CHEMBL3128919) Inhibition of HIF-1 alpha in human OVCAR3 cells by Western blotting analysis in hypoxia condition ChEMBL_1294797 (CHEMBL3128920) Inhibition of HIF-1 alpha in human PANC1 cells by Western blotting analysis in hypoxia condition ChEMBL_458038 (CHEMBL924307) Inhibition of hypoxia induced HIF1-alpha transcriptional activity in human Hep3B cells by reporter gene assay ChEMBL_458039 (CHEMBL924308) Inhibition of hypoxia induced HIF1-alpha transcriptional activity in human AGS cells by reporter gene assay ChEMBL_469602 (CHEMBL933161) Inhibition of hypoxia-induced HIF1 transcriptional activation in human U251-HRE cells by luciferase reporter assay ChEMBL_478428 (CHEMBL932617) Inhibition of hypoxia-induced HIF1 activation in human T47D cells by cell based reporter gene assay ChEMBL_1294792 (CHEMBL3128879) Inhibition of Stat3 phosphorylation in human MDA-MB-468 cells by Western blotting analysis in hypoxia condition ChEMBL_1294793 (CHEMBL3128880) Inhibition of Stat3 phosphorylation in human MDA-MB-231 cells by Western blotting analysis in hypoxia condition ChEMBL_2281079 Inhibition of hypoxia-induced HIF1alpha/P300 interaction (unknown origin) incubated for 30 mins for fluorescence anisotropy competition assay ChEMBL_428795 (CHEMBL916228) Inhibition of hypoxia induced HIF1 transcriptional activity in human Hep3B cells by cell-based HRE reporter assay ChEMBL_458037 (CHEMBL924306) Inhibition of hypoxia induced HIF1-alpha transcriptional activity in human Sk-Hep1 cells by reporter gene assay ChEMBL_573857 (CHEMBL1056199) Inhibition of hypoxia-induced HIF1 activation in human Hep3B cells by pGL3-HRE-luciferase reporter gene assay ChEMBL_619005 (CHEMBL1101830) Inhibition of hypoxia-induced HIF1 activation in human T47D cells by pTK-HRE3-luciferase reporter gene assay ChEMBL_698465 (CHEMBL1647397) Inhibition of hypoxia-induced HIF1 activation in human T47D cells by HRE3-TK-luciferase reporter gene assay ChEMBL_698468 (CHEMBL1647400) Inhibition of hypoxia-induced HIF1 activation in human PC3 cells by HRE3-TK-luciferase reporter gene assay ChEMBL_770820 (CHEMBL1837625) Inhibition of hypoxia-induced HIF1alpha activation in human ME180 cells by HRE3-Bla-luciferase reporter gene assay ChEMBL_881925 (CHEMBL2212528) Inhibition of HIF-1alpha in human HCT116 cells under hypoxia condition by HRE-luciferase reporter gene assay ChEMBL_881926 (CHEMBL2212529) Inhibition of HIF-1alpha in human LN229 cells under hypoxia condition by HRE-luciferase reporter gene assay ChEMBL_1294798 (CHEMBL3128921) Inhibition of HIF-1 alpha in human MDA-MB-468 cells by Western blotting analysis in hypoxia condition ChEMBL_1294799 (CHEMBL3128922) Inhibition of HIF-1 alpha in human MDA-MB-231 cells by Western blotting analysis in hypoxia condition ChEMBL_452324 (CHEMBL901549) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by cell based luciferase assay ChEMBL_550357 (CHEMBL999911) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by luciferase reporter gene assay ChEMBL_753564 (CHEMBL1799182) Inhibition of hypoxia-induced HIF1alpha activation in human Hep3B cells after 16 hrs by HRE-luciferase reporter assay ChEBML_1685544 Inhibition of hypoxia-induced HIF1alpha (unknown origin) transcriptional activity expressed in human HCT116 cells by HRE luciferase reporter gene assay ChEMBL_610892 (CHEMBL1065047) Inhibition of hypoxia-induced HIF1alpha activation in human HeLa cells after 12 hrs by HRE-luciferase reporter gene assay ChEMBL_1627457 (CHEMBL3869978) Inhibition of hypoxia-induced HIF1 transcriptional activity in human HeLa cells measured after 12 hrs by luciferase reporter gene assay ChEMBL_481732 (CHEMBL1002833) Inhibition of hypoxia-induced HIF1 activation in human T47D cells carrying pTK-HER3-luc reporter gene measured by luciferase activity ChEMBL_526045 (CHEMBL967263) Inhibition of hypoxia-induced HIF1 activation in human AGS cells after 16 hrs by pGL3-HRE-luciferase reporter gene assay ChEMBL_549581 (CHEMBL1015932) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by pTK-HRE3-luciferase reporter gene assay ChEMBL_621709 (CHEMBL1104951) Inhibition of hypoxia-induced HIF1 activation in human PC3 cells after 16 hrs by pTK-HRE3-luciferase reporter gene assay ChEMBL_621727 (CHEMBL1108459) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by pTK-HRE3-luciferase reporter gene assay ChEMBL_621731 (CHEMBL1108463) Inhibition of hypoxia-induced HIF1 activation in human T47D cells expressing pGL3 construct after 16 hrs by reporter gene assay ChEMBL_776653 (CHEMBL1913750) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay ChEMBL_851302 (CHEMBL2155888) Inhibition of hypoxia-induced HIF1 activation in human T47D cells after 16 hrs by HRE3-TK-luciferase reporter gene assay ChEMBL_2288784 Inhibition of DHODH in human HeLa cells assessed as reduction in hypoxia-induced VEGF expression incubated for 48 hrs by ELISA assay ChEMBL_631037 (CHEMBL1113762) Inhibition of hypoxia-induced HIF1 activation in human T47D cells in presence of 1% O2 by HRE3-TK-luc reporter assay ChEMBL_2375999 Inhibition of HIF-1 alpha transcriptional activity in human U-251 cells incubated for 24 hrs under hypoxia condition by luciferse reporter assay ChEMBL_439940 (CHEMBL890257) Inhibition of HIF1 activation in human AGS cells assessed as inhibition of hypoxia-induced luciferase expression after 16 hrs by reporter assay ChEMBL_439941 (CHEMBL890258) Inhibition of HIF1 activation in human Hep3B cells assessed as inhibition of hypoxia-induced luciferase expression after 16 hrs by reporter assay ChEMBL_735620 (CHEMBL1693322) Inhibition of hypoxia-induced HIF1 activation in human T47D cells expressing pGL3 construct after 16 hrs by cell-based luciferase reporter assay ChEMBL_938618 (CHEMBL2327238) Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human HeLa cells expressing HRE-Luc after 12 hrs by luciferase reporter gene assay Evaluation of Inhibitory Activity Against Retinal Neovascularization by Hypoxia The experiment was performed in order to evaluate inhibitory activity against angiogenesis of the compounds. ChEMBL_573855 (CHEMBL1055397) Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis ChEMBL_631036 (CHEMBL1113761) Inhibition of chemical hypoxia-induced HIF1 activation in human T47D cells in presence of 1, 10-phenanthrolin by HRE3-TK-luc reporter assay ChEMBL_636313 (CHEMBL1169036) Inhibition of hypoxia-induced HIF1alpha protein accumulation in human Hep3B cells treated for 30 mins measured after 12 hrs by Western blot analysis ChEMBL_1855371 (CHEMBL4356100) Inhibition of HIF1alpha transcriptional activity in hypoxia-induced human HCCLM3 cells co-transfected with luciferase reporter plasmid containing five copies of HREs and pRL-SV40 plasmid encoding Renilla luciferase incubated with compound under normoxia for 1 hr followed by hypoxia induction and measured after 24 hrs by HRE-dependent dual luciferase reporter gene assay ChEBML_66293 The compound was tested for binding affinity to FK506 binding protein 12 using Rapamycin as control, with an ascomycin conjugate of alkaline phosphatase in a competition binding assay ChEMBL_1891621 (CHEMBL4393448) Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells after 24 hrs in hypoxic condition by HRE-dependent dual luciferase reporter gene assay ChEMBL_66293 (CHEMBL678815) The compound was tested for binding affinity to FK506 binding protein 12 using Rapamycin as control, with an ascomycin conjugate of alkaline phosphatase in a competition binding assay ChEMBL_1766949 (CHEMBL4202196) Inhibition of HIF-1alpha in human HRE-A549 cells pre-incubated for 1 hr before exposure to hypoxia for 24 hrs by HRE-luciferase reporter gene assay ChEMBL_881913 (CHEMBL2212516) Inhibition of topoisomerase-1 in human U251 cells assessed as inhibition of HIF-1-mediated hypoxia-induced VEGF expression after 24 hrs by luciferase reporter gene assay ChEMBL_881914 (CHEMBL2212517) Inhibition of topoisomerase-1 in human U251 cells assessed as inhibition of hypoxia-induced HIF-1alpha accumulation in nuclear extract after 6 to 24 hrs by immunoblot analysis ChEMBL_2080981 (CHEMBL4736772) Binding affinity to FKBP12 (unknown origin) expressed in HEK293 cells co-expressing FRB assessed as inhibition of rapamycin-induced FKBP12-FRB dimerization measured after 25 mins by nano-glo live cell reagent based luminescence assay ChEMBL_1465276 (CHEMBL3406495) Inhibition of HIF1 in human HT1080 cells transfected with 5xHRE/pGL3/VEGF/E1b reporter plasmid pre-incubated for 1 hr followed by incubation under hypoxia conditions for 24 hrs by HRE-driven luciferase reporter gene assay ChEMBL_860195 (CHEMBL2168935) Inhibition of P300 CH1 domain-mediated HIF1 transcriptional activity in human LN229-V6R cells assessed as reduction in luciferase activity incubated for 1 hr under normoxia followed by 24 hrs under hypoxia by reporter gene assay ChEMBL_829292 (CHEMBL2060139) Inhibition of HIF1 transcriptional activity in human LN229 cells expressing VEGF-HRE-V6R and coexpressing luciferase, lac Z gene incubated for 1 hr in normoxia condition followed by 24 hrs in hypoxia condition by reporter gene assay ChEMBL_2050089 (CHEMBL4704788) Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells co-transfected with luciferase reporter plasmid containing six copies of HREs and pRL-CMV vector assessed as reduction in luciferase activity incubated for 24 hrs under hypoxic condition by HRE-dependent dual luciferase reporter gene assay ChEMBL_2102766 (CHEMBL4811162) Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed as reduction in luciferase activity incubated for 1 hrs under normoxic condition followed by incubation under hypoxic condition for 24 hrs by luciferase reporter gene assay ChEMBL_2128544 (CHEMBL4837973) Inhibition of hypoxia-induced HIF1alpha transcriptional activity in human Hep3B cells co-transfected with luciferase reporter plasmid containing six copies of HREs and pRL-CMV vector assessed as reduction in luciferase activity incubated for 24 hrs under hypoxic condition by HRE-dependent dual luciferase reporter gene assay Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically AGP1 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically CIT2 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although th Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically LAP4 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically MEP2 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically RPL19A University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t mTOR Assay Mammalian target of rapamycin (mTOR) was assayed by monitoring phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence resonance energy transfer format. The mTOR-mediated phosphorylation was measured under initial rate conditions. After incubation, the phosphorylated substrate was detected with Tb-anti-p4E-BP1 antibody before reading on a Perkin-Elmer EnVision Fluorescence Reader (exc 340; em 495/520). Duplicate dose-response curves were fit to an equation of competitive tight-binding inhibition. Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically AGP1 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically CIT2 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically LAP4 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically MEP2 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically RPL19A based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth In Vitro Inhibition Assay The Invitrogen (Carlsbad, Calif.) mammalian target of rapamycin (mTOR) Lanthascreen assay can be used to quantitate mTOR kinase activity in an in vitro setting. Active mTOR phosphorylates eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) on residue threonine 46. This phosphorylation event can be detected with a phospho-specific terbium (Tb) labeled Ab, in turn bringing the Tb label in close proximity to the GFP tagged 4E-BP1 and allowing for time-resolved fluorescence resonance energy transfer (TR-FRET), which correlates 4E-BP1 phosphorylation levels with mTOR kinase activity. In Vitro mTOR Assay The Invitrogen (Carlsbad, Calif.) mammalian target of rapamycin (mTOR) Lanthascreen assay can be used to quantitate mTOR kinase activity in an in vitro setting. Active mTOR phosphorylates eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) on residue threonine 46. This phosphorylation event can be detected with a phospho-specific terbium (Tb) labeled Ab, in turn bringing the Tb label in close proximity to the GFP tagged 4E-BP 1 and allowing for time-resolved fluorescence resonance energy transfer (TR-FRET), which correlates 4E-BP1 phosphorylation levels with mTOR kinase activity. SAR analysis for the identification of translation initiation inhibitors (PABP) Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Number: 1R03MH084835-01 Assay Provider: Jerry Pelletier, Ph.D, McGill University, Montreal, Canada Translation is an essential cellular process whose deregulation is associated with alterations in cell growth, cell cycle progression, and cell death responses. The initiation phase of translation is a key target for regulation when cells are exposed to various environmental cues (e.g. insulin, amino acid starvation, mitogenic stimulation, hypoxia, etc). As well, translation initiation control is usurped upon viral infection and is deregulated in many human cancers. Over-expression of certain translation factors can lead to malignant transformation and many of the components of the translational apparatus are over-expressed in human cancers. Several tumor sup SAR analysis for the identification of translation initiation inhibitors (eIF4H) Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBIMR, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Number: 1R03MH084835-01 Assay Provider: Jerry Pelletier, Ph.D, McGill University, Montreal, Canada Translation is an essential cellular process whose deregulation is associated with alterations in cell growth, cell cycle progression, and cell death responses. The initiation phase of translation is a key target for regulation when cells are exposed to various environmental cues (e.g. insulin, amino acid starvation, mitogenic stimulation, hypoxia, etc). As well, translation initiation control is usurped upon viral infection and is deregulated in many human cancers. Over-expression of certain translation factors can lead to malignant transformation and many of the components of the translational apparatus are over-expressed in human cancers. Several tumor su uHTS fluorescence polarization assay for the identification of translation initiation inhibitors (PABP) Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Number: 1R03MH084835-01 Assay Provider: Jerry Pelletier, Ph.D, McGill University, Montreal, Canada Translation is an essential cellular process whose deregulation is associated with alterations in cell growth, cell cycle progression, and cell death responses. The initiation phase of translation is a key target for regulation when cells are exposed to various environmental cues (e.g. insulin, amino acid starvation, mitogenic stimulation, hypoxia, etc). As well, translation initiation control is usurped upon viral infection and is deregulated in many human cancers. Over-expression of certain translation factors can lead to malignant transformation and many of the components of the translational apparatus are over-expressed in human cancers. Several tumor suppresso uHTS fluorescence polarization assay for the identification of translation initiation inhibitors (eIF4H) Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Number: 1R03MH084835-01 Assay Provider: Jerry Pelletier, Ph.D, McGill University, Montreal, Canada Translation is an essential cellular process whose deregulation is associated with alterations in cell growth, cell cycle progression, and cell death responses. The initiation phase of translation is a key target for regulation when cells are exposed to various environmental cues (e.g. insulin, amino acid starvation, mitogenic stimulation, hypoxia, etc). As well, translation initiation control is usurped upon viral infection and is deregulated in many human cancers. Over-expression of certain translation factors can lead to malignant transformation and many of the components of the translational apparatus are over-expressed in human cancers. Several tumor suppresso PI3K Inhibition Assay PI3K inhibition assay (PI3K Assay (Emmanuelle M, Huang Y, Yan H G et al. Targeting Protein Translation in Human Non-Small Cell Lung Cancer via Combined MEK and Mammalian Target of Rapamycin Suppression. Cancer Res 67:(23). (2007).) was carried out by PI3 Kinase activity/inhibitor assay kit, where PI3 kinase reaction was set up in Glutathione-coated strips/plate for inhibitor reaction. Kinase and inhibitors were pre-incubated for 10 minutes prior to the addition of PIP2 substrate. 5 uL of 5x kinase reaction buffer were added in each well followed by the further addition of 5 uL/well of PIP2 substrate. Then distilled H2O was added to each well so as to make up a final volume of 25 uL/well. Incubation was done at rt for 1 hour which was followed by washing the wells 3 times with 200 uL of 1xTBST per well and then 2 times with 200 uL of IX TBS per well. Then 100 uL of the Substrate TMB per well was added and then to keep for colour development in the dark. SPR Assay to Determine Binding Affinity to FKBP51 Biotinylated avi-FKBP51 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 2000 RU, 3 μg/ml biotinylated avi-FKBP51 were injected for 360 sec at a flow rate of 10 μl/min. Rapalogs were diluted in DMSO to 100× working concentration. Each Rapalog was 100-fold diluted in 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20 and a serial dilution prepared (8 concentrations, 3-fold dilutions, 0.5-1000 nM). Rapamycin was used as reference sample (8 concentrations, 3-fold dilutions, 0.5-1000 nM). The compound dilutions were then injected at 100 uL/min for 120 seconds contact time and with 3600 seconds dissociation time with increasing concentrations. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer. Multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). SPR Assay to Determine Binding Affinity to FKBP12 Biotinylated avi-FKBP12 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 1000 RU, 2 μg/ml biotinylated avi-FKBP12 were injected for 100 sec at a flow rate of 10 μl/min. Rapalogs were diluted in DMSO to 100× working concentration. Each Rapalog was 100-fold diluted in 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20 and a serial dilution prepared (9 concentrations, 3-fold dilutions, 0.08-500 nM). Rapamycin was used as reference sample (9 concentrations, 3-fold dilutions, 0.02-100 nM). The compound dilutions were then injected at 100 uL/min for 120 seconds contact time in sequence with increasing concentrations. Dissociation was monitored for 3600 seconds. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer. The single-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). SPR Assay to Characterize Ternary Complex Formation with FKBP12 Biotinylated avi-FKBP12 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 100 RU, 0.3 g/ml biotinylated avi-FKBP12 were injected for 80 sec at a flow rate of 10 l/min. Serial dilution of FRB was prepared (12 concentrations, 3-fold dilutions, 0.00011-20 M) and supplemented with 100 nM of rapalog. A-B-A injection mode was used to ensure saturation immobilized FKBP12 with respective rapalog. 100 nM solution of the respective rapalog was injected before FRB injection for 120 sec and during dissociation for 420 sec. The FRB dilutions were then injected 120 seconds contact time with increasing concentrations. Rapamycin was used as reference sample. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer at a flow rate of 30 l/min. The multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). In case of fast association and dissociation, steady state affinity analysis following the law of mass action was used to determine the affinity Kd (M). SPR Assay to Characterize Ternary Complex Formation with FKBP51 Biotinylated avi-FKBP51 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 200 RU, 0.6 g/ml biotinylated avi-FKBP51 were injected for 150 sec at a flow rate of 10 l/min. Serial dilution of FRB was prepared (12 concentrations, 3-fold dilutions, 0.00011-20 M) and supplemented with 100 nM of rapalog. A-B-A injection mode was used to ensure saturation immobilized FKBP12 with respective rapalog. 100 nM solution of the respective rapalog was injected before FRB injection for 120 sec and during dissociation for 420 sec. The FRB dilutions were then injected 120 seconds contact time with increasing concentrations. Rapamycin was used as reference sample. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer at a flow rate of 30 l/min. The multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). In case of fast association and dissociation, steady state affinity analysis following the law of mass action was used to determine the affinity Kd (M). Inhibitory Effect of the Compounds on PHD2 The interaction between hypoxia-inducible factor HIF-1α and VBC complex (von Hippel-Lindau protein-Elongin B-Elongin C, VBC) was detected by Fluorescence polarization (FP) method, to measure the enzyme inhibitory activity of the HIF Prolyl hydroxylases 2 (PHD2) inhibitor compounds.To a NETN (20 mM Tris.HCl, 100 mM NaCl, 1 mM EDTA, 0.5% NP-40, 1 mM PMSF) buffer containing 200 μM ascorbic acid, 20 μM α-ketoglutaric acid, 100 μM FeCl2 was added FAM-HIF (556-575) at a final concentration of 1 μM in the dark. Subsequently, the desired concentration of the test compound or the positive compound was added (the compound was replaced by the buffer in the negative control and the positive control). Finally, PHD2 was added at a final concentration of 0.5 μg/μl (PHD2 was replaced by the buffer in the negative control). They were mixed well and allowed to stand at room temperature for 30 minutes in the dark followed by 95° C. water bath for 1 minute, and then the reaction was terminated. After the temperature drops to room temperature, the sample was prepared well for use. EBC buffer (50 mM Tris.HCl, 120 mM NaCl, 0.5% NP-40) was added to the corresponding wells of a black 96-well test plate. A GST-VBC complex was added to the corresponding test wells at a final concentration of 300 nM (using the wells containing only EBC buffer as blank wells). Subsequently, the corresponding PHD2 prolyl hydroxylation reaction sample was added in the dark as a substrate with a final concentration of 100 nM. After mixing well, the lateral and longitudinal fluorescence intensity values were measured using a full-wavelength multifunctional microplate reader (TECAN infinite M1000) at an excitation wavelength of 407 nm and an emission wavelength of 518 nm.
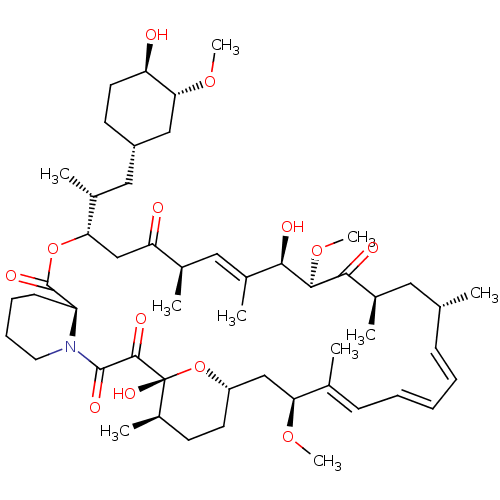 BDBM92863 mTOR Inhibitor, Rapamycin US9505773, Rapamycin
BDBM92863 mTOR Inhibitor, Rapamycin US9505773, Rapamycin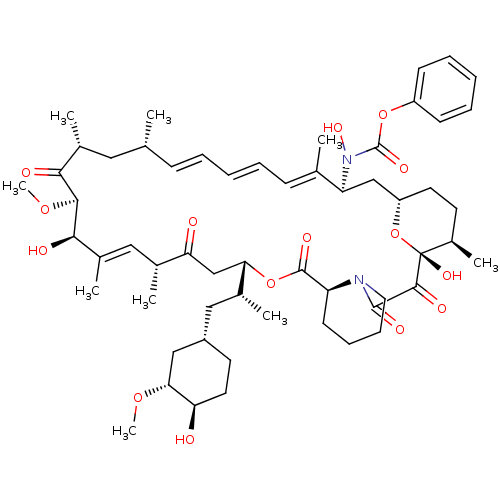 CHEMBL437064 Rapamycin analogue BDBM50089437
CHEMBL437064 Rapamycin analogue BDBM50089437 Rapamycin analogue CHEMBL281297 BDBM50089447
Rapamycin analogue CHEMBL281297 BDBM50089447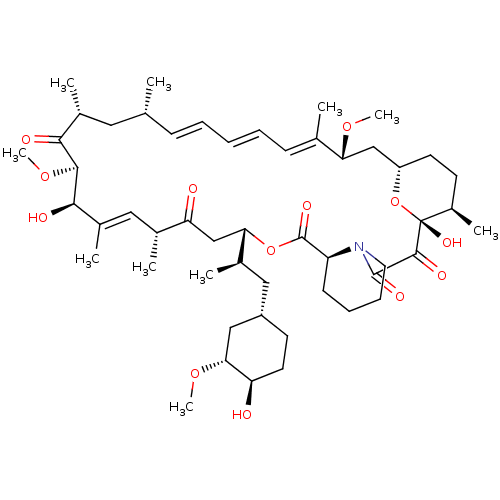 BDBM36608 Rapamycin C-7, analog 1
BDBM36608 Rapamycin C-7, analog 1 BDBM36610 Rapamycin C-7, analog 5a
BDBM36610 Rapamycin C-7, analog 5a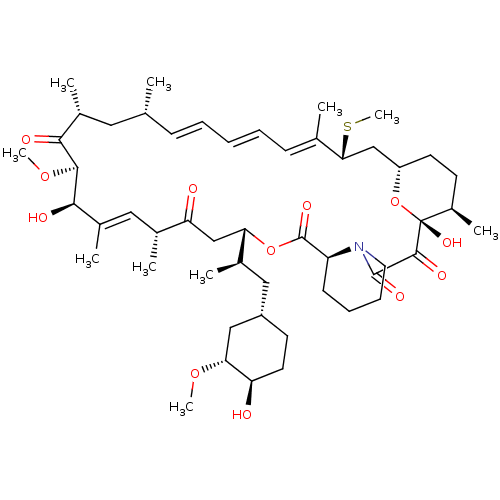 BDBM36612 Rapamycin C-7, analog 6a
BDBM36612 Rapamycin C-7, analog 6a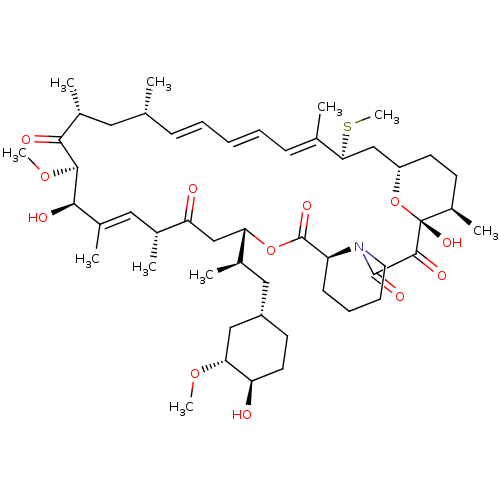 BDBM36613 Rapamycin C-7, analog 6b
BDBM36613 Rapamycin C-7, analog 6b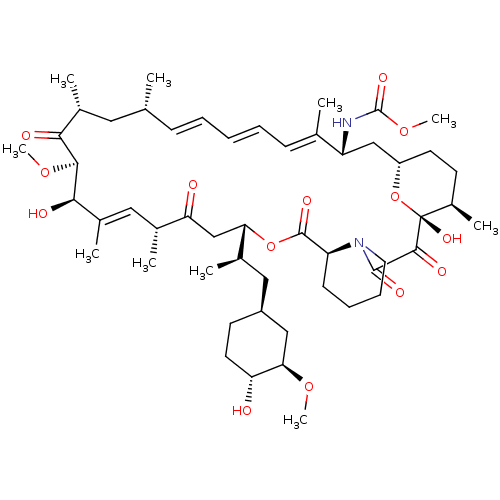 BDBM36614 Rapamycin C-7, analog 7a
BDBM36614 Rapamycin C-7, analog 7a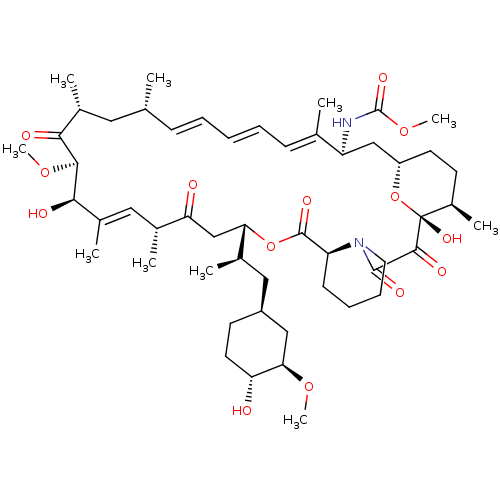 BDBM36615 Rapamycin C-7, analog 7b
BDBM36615 Rapamycin C-7, analog 7b BDBM36616 Rapamycin C-7, analog 8a
BDBM36616 Rapamycin C-7, analog 8a BDBM36617 Rapamycin C-7, analog 8b
BDBM36617 Rapamycin C-7, analog 8b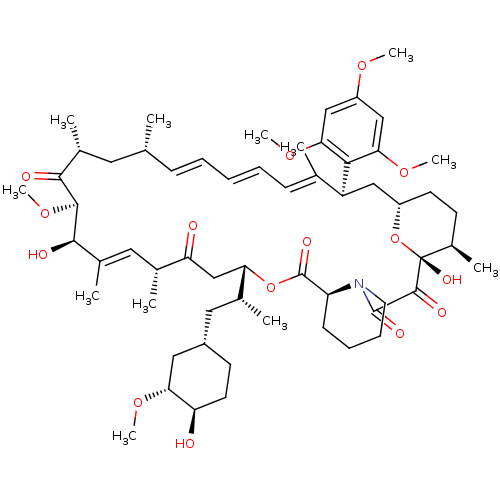 BDBM36618 Rapamycin C-7, analog 9
BDBM36618 Rapamycin C-7, analog 9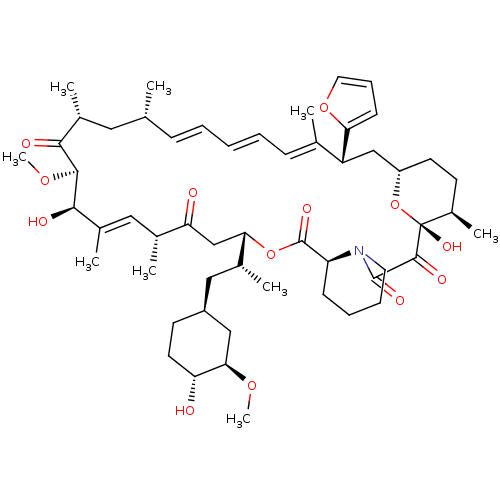 BDBM36619 Rapamycin C-7, analog 10a
BDBM36619 Rapamycin C-7, analog 10a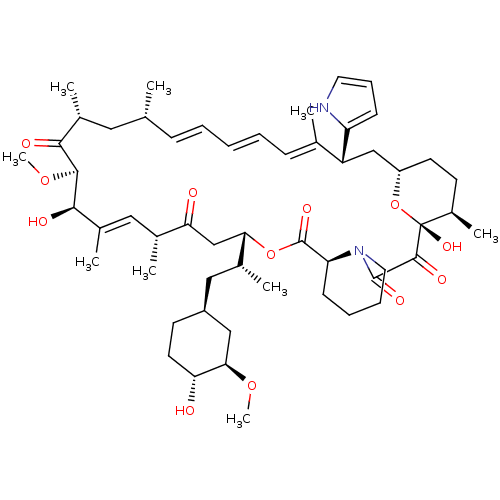 BDBM36621 Rapamycin C-7, analog 11a
BDBM36621 Rapamycin C-7, analog 11a BDBM36622 Rapamycin C-7, analog 11b
BDBM36622 Rapamycin C-7, analog 11b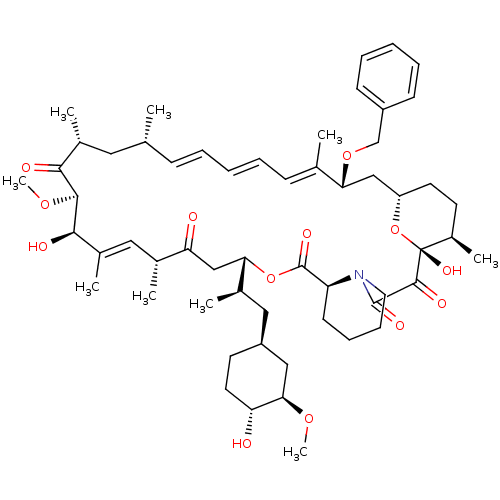 BDBM36625 Rapamycin C-7, analog 14a
BDBM36625 Rapamycin C-7, analog 14a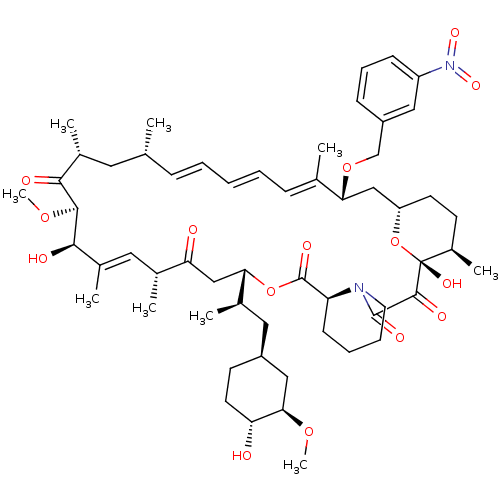 BDBM36626 Rapamycin C-7, analog 15a
BDBM36626 Rapamycin C-7, analog 15a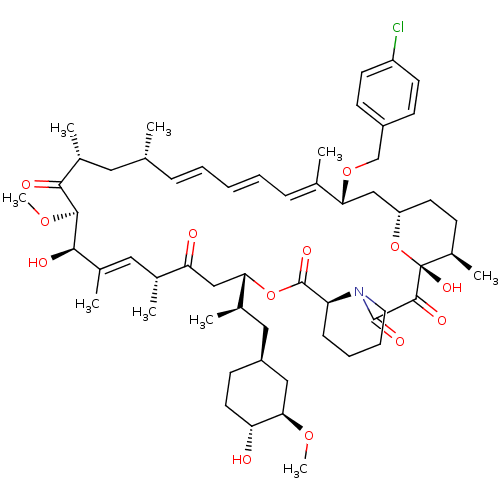 BDBM36627 Rapamycin C-7, analog 16a
BDBM36627 Rapamycin C-7, analog 16a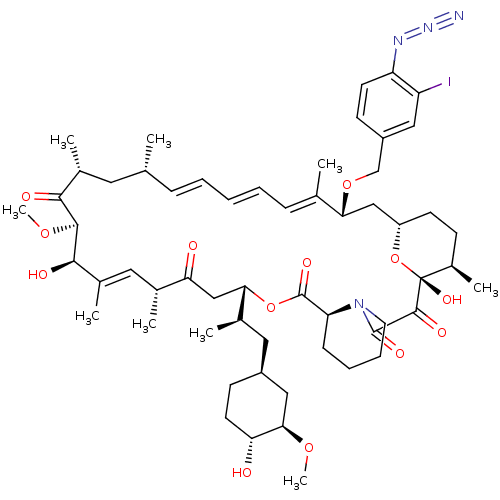 BDBM36628 Rapamycin C-7, analog 17a
BDBM36628 Rapamycin C-7, analog 17a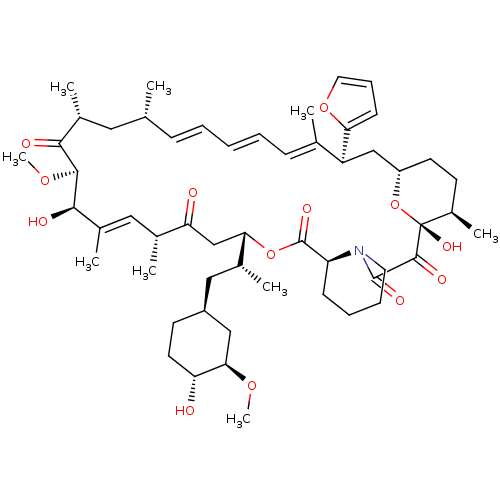 Rapamycin C-7, analog 10b BDBM36620
Rapamycin C-7, analog 10b BDBM36620 Rapamycin C-7, analog 12 BDBM36623
Rapamycin C-7, analog 12 BDBM36623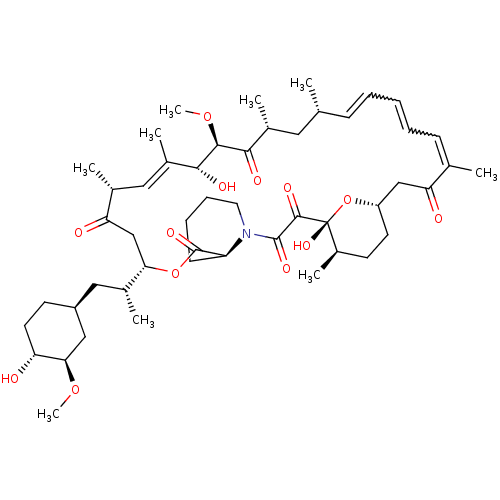 Rapamycin C-7, analog 13 BDBM36624
Rapamycin C-7, analog 13 BDBM36624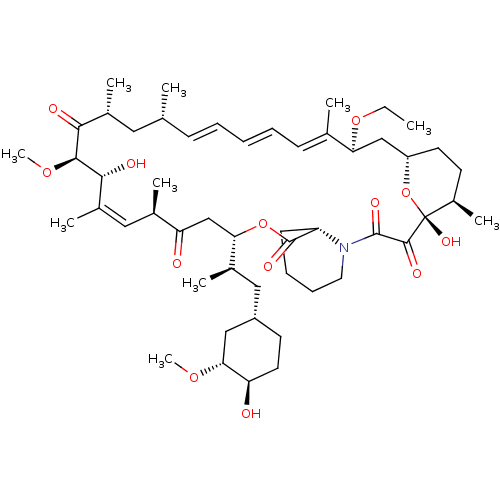 Rapamycin C-7, analog 5b BDBM36611
Rapamycin C-7, analog 5b BDBM36611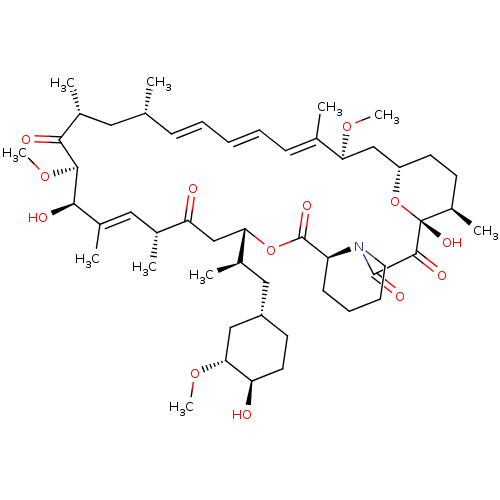 Rapamycin C-7, analog 4 BDBM36609 US11603377, Compound Ramycin SIROLIMUS
Rapamycin C-7, analog 4 BDBM36609 US11603377, Compound Ramycin SIROLIMUS BDBM757304 (S)-C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-rapamycin US20250223297, Example 1
BDBM757304 (S)-C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-rapamycin US20250223297, Example 1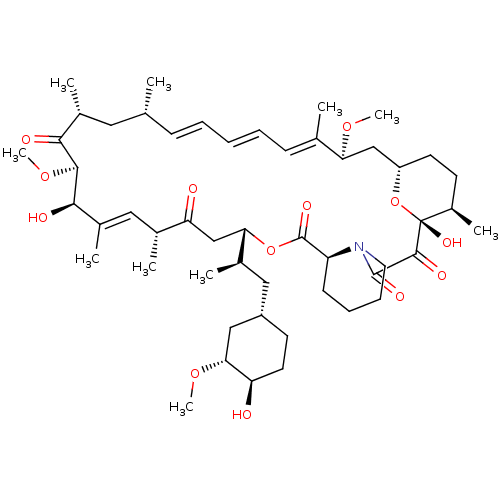 Sirolimus BDBM50064359 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (Rapamycin) Rapamune Rapamycin AY-22989
Sirolimus BDBM50064359 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (Rapamycin) Rapamune Rapamycin AY-22989 US12091424, Example 14 C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1) BDBM696419
US12091424, Example 14 C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1) BDBM696419 US12091424, Example 6 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-rapamycin (Diastereomer 1) US20250223297, Example 6 BDBM696416
US12091424, Example 6 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-rapamycin (Diastereomer 1) US20250223297, Example 6 BDBM696416 US20250223297, Example 10 US12091424, Example 10 BDBM696418 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1)
US20250223297, Example 10 US12091424, Example 10 BDBM696418 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1)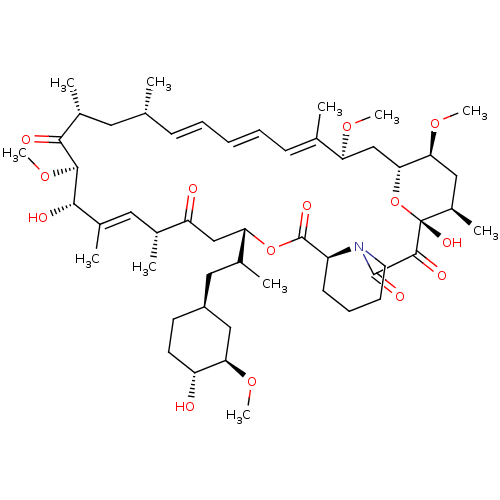 FK-506-M CHEMBL413 Rapamycin (16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2 RAPAMYCIN IMMUNOSUPPRESSANT DRUG BDBM50068561 WY-090217 Rapamune Sirolimus analogue AY-22989 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30,33-trimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;Rapamycin 1,18-dihydroxy-12-{2-[4-hydroxy-3-methoxy-(4R)-cyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone SIROLIMUS
FK-506-M CHEMBL413 Rapamycin (16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2 RAPAMYCIN IMMUNOSUPPRESSANT DRUG BDBM50068561 WY-090217 Rapamune Sirolimus analogue AY-22989 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30,33-trimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;Rapamycin 1,18-dihydroxy-12-{2-[4-hydroxy-3-methoxy-(4R)-cyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone SIROLIMUS