Query String: T3
 US9878991, Compound T3 BDBM236160 US9365563, T3
US9878991, Compound T3 BDBM236160 US9365563, T3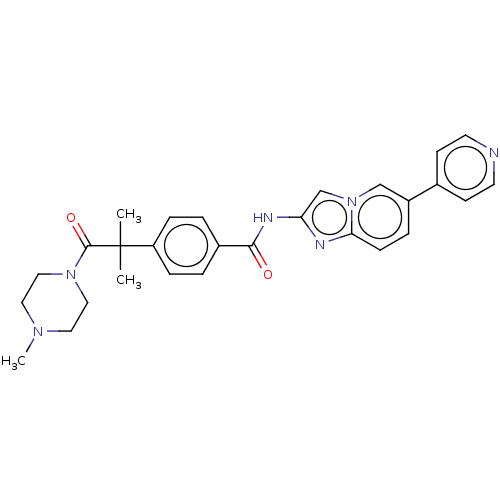 BDBM50592246 T3-CLK
BDBM50592246 T3-CLK BDBM511489 US11059792, Compound T3
BDBM511489 US11059792, Compound T3 BDBM546519 US11292781, Compound T3
BDBM546519 US11292781, Compound T3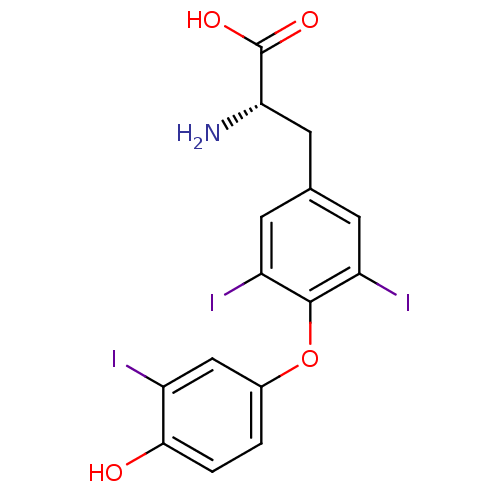 [125I]T3 US10544075, Compound T3 CHEMBL1544 triothyrone Triiodothyronine Triiodothyronine (T3) 3,5,3'-triiodo-L-thyronine (T3) (2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid BDBM18860 liothyronine
[125I]T3 US10544075, Compound T3 CHEMBL1544 triothyrone Triiodothyronine Triiodothyronine (T3) 3,5,3'-triiodo-L-thyronine (T3) (2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid BDBM18860 liothyronine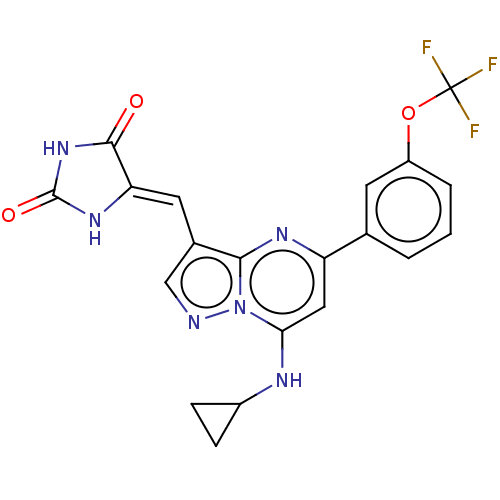 US9303033, T3, Table 13A, Compound 2 BDBM218913 US9303033, H7, Table 22A, Compound 2
US9303033, T3, Table 13A, Compound 2 BDBM218913 US9303033, H7, Table 22A, Compound 2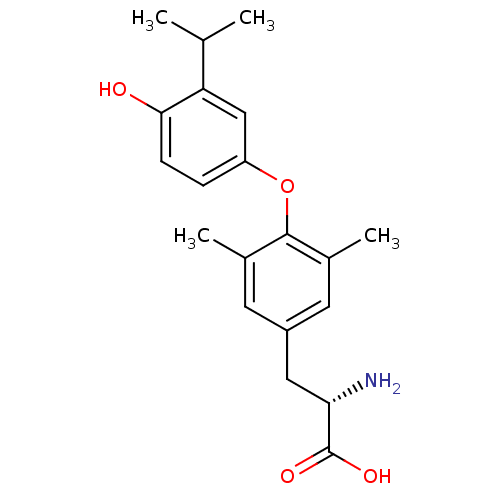 CHEMBL105983 BDBM50130595 2-Amino-3-[4-(4-hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-phenyl]-propionic acid Dimethyl-isopropyl-T3 (DIMIT)
CHEMBL105983 BDBM50130595 2-Amino-3-[4-(4-hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-phenyl]-propionic acid Dimethyl-isopropyl-T3 (DIMIT) US10087178, Example T3 BDBM288135 (1S,2S)-N-(2,4- difluorobenzyl)-9'-hydroxy-2- (methoxymethyl)-2'-methyl- 1',8'-dioxo-1',2',3',8'- tetrahydrospiro[cyclopropane- 1,4'-pyrido[1,2-a]pyrazine]- 7'-carboxamide hydrochloride
US10087178, Example T3 BDBM288135 (1S,2S)-N-(2,4- difluorobenzyl)-9'-hydroxy-2- (methoxymethyl)-2'-methyl- 1',8'-dioxo-1',2',3',8'- tetrahydrospiro[cyclopropane- 1,4'-pyrido[1,2-a]pyrazine]- 7'-carboxamide hydrochloride
- ChEMBL_471200 (CHEMBL939179) Displacement of [125I]T3 from human TRalpha receptor
- ChEMBL_471201 (CHEMBL939180) Displacement of [125I]T3 from human TRbeta receptor
- ChEMBL_210505 (CHEMBL811900) Inhibitory activity against [125I]T3 binding to human TRbeta1 receptor
- ChEMBL_210491 (CHEMBL814598) Binding affinity against human Thyroid hormone receptor alpha-1 (hTRalpha1) using radiolabeled T3
- ChEMBL_210502 (CHEMBL811897) Binding affinity against human Thyroid hormone receptor beta 1 (hTRbeta1) using radiolabeled T3
- ChEMBL_143914 (CHEMBL872845) In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor
- ChEMBL_157265 (CHEMBL765465) In vitro inhibition of bound [125I]L-T3 rat plasma membrane 3,5,3'' L-triiodothyronine receptor
- ChEMBL_143915 (CHEMBL752718) Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro.
- ChEMBL_533636 (CHEMBL969512) Displacement of [125I]T3 from recombinant thyroid hormone receptor alpha expressed in sf9 cells by scintillation proximity assay
- ChEMBL_533637 (CHEMBL969513) Displacement of [125I]T3 from recombinant thyroid hormone receptor beta expressed in sf9 cells by scintillation proximity assay
- TRalpha-Binding Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha.
- TRbeta-Binding Assay IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta.
- ChEMBL_683506 (CHEMBL1286421) Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at monophasic kinetics
- ChEMBL_683507 (CHEMBL1286422) Inhibition of Candida tropicalis T3 blood stream isolate glucan synthase subunit FKS1p with FLTLS/PLRDP mutant at biphasic kinetics
- ChEMBL_935146 (CHEMBL2317308) Displacement of [125I]-T3 from human TRbeta expressed in insect cells after 16 to 48 hrs by gamma-counting
- ChEMBL_935147 (CHEMBL2317309) Displacement of [125I]-T3 from human TRalpha expressed in insect cells after 16 to 48 hrs by gamma-counting
- ChEMBL_821529 (CHEMBL2038197) Displacement of [125I]T3 from human recombinant thyroid harmone receptor beta after 16 to 48 hrs by gamma-ray detection
- ChEMBL_821530 (CHEMBL2038198) Displacement of [125I]T3 from human recombinant thyroid harmone receptor alpha after 16 to 48 hrs by gamma-ray detection
- ChEMBL_210489 (CHEMBL814596) Concentration required to inhibit 50% of binding of [125I]-T3 to human Thyroid hormone receptor alpha-1 in CHO-K1 cells
- ChEMBL_210500 (CHEMBL811895) Concentration required to inhibit 50% of binding of [125I]T3 to human Thyroid hormone receptor beta 1 in CHO-K1 cells
- TRalpha-Binding Assay and Thyroid Response Element (TRAFalpha1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to inhibit 50% of T3-induced TRalpha-activation in CHOK1 cells stably transfected with hTRalpha using alkaline phosphatase reporter assay.
- TRalpha-Binding Assay and Thyroid Response Element (TRAFalpha1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to reach 50% of T3-induced TRalpha-activation in CHOK1 cells stably transfected with hTRalpha using alkaline phosphatase reporter assay.
- TRalpha-Binding Assay and Thyroid Response Element (TRAFalpha1) Reporter Assay. IIC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to inhibit 50% of T3-induced TRalpha-activation in CHOK1 cells stably transfected with hTRalpha using alkaline phosphatase reporter assay.
- TRbeta-Binding Assay and Thyroid Response Element (TRAFbeta1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to inhibit 50% of T3-induced TRbeta-activation in CHOK1 cells stably transfected with hTRbeta using alkaline phosphatase reporter assay.
- TRbeta-Binding Assay and Thyroid Response Element (TRAFbeta1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to reach 50% of T3-induced TRbeta-activation in CHOK1 cells stably transfected with hTRbeta using alkaline phosphatase reporter assay.
- TRalpha-Binding Assay and Thyroid Response Element (TRAFalpha1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to reach or inhibit (agonist or antagonist) 50% of T3-induced TRalpha-activation in CHOK1 cells stably transfected with hTRalpha using alkaline phosphatase reporter assay.
- TRbeta-Binding Assay and Thyroid Response Element (TRAFbeta1) Reporter Assay. IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to reach or inhibit (agonist or antagonist) 50% of T3-induced TRbeta-activation in CHOK1 cells stably transfected with hTRbeta using alkaline phosphatase reporter assay.
- ChEMBL_1750174 (CHEMBL4184934) Antagonist activity at CMV-fused TRalpha-LBD-GAL4-DBD (unknown origin) expressed in HEK293T cells assessed as inhibition of T3-induced receptor transactivation after 16 hrs by bright-Glo luciferase reporter gene assay
- ChEMBL_1750175 (CHEMBL4184935) Antagonist activity at CMV-fused TRbeta-LBD-GAL4-DBD (unknown origin) expressed in HEK293T cells assessed as inhibition of T3-induced receptor transactivation after 16 hrs by bright-Glo luciferase reporter gene assay
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay for Thyroid Hormone Receptor Agonist Screening LanthaScreen TR-FRET Thyroid Receptor alpha Coactivator Assay kit (ThermoFisher) and LanthaScreen TR-FRET Thyroid Receptor beta Coactivator Assay kit (ThermoFisher) were used for agonist compound screening. Compounds in DMSO were diluted using ECHO Liquid Handler (Labcyte Inc.) into 384 plates in 10-point 3-fold series in duplicate (5 micro M final top concentration). Buffer C (ThermoFisher) was added to each well before the 4 mixture of fluorescein-SCR2-2 coactivator (200 nM final concentration), Terbium-labeled anti-GST antibody (2 nM final concentration), and TR alpha-LBD (0.4 nM final concentration) or TR beta-LBD (1.0 nM final concentration) was added. After 2 hour incubation at room temperature in dark, the TR-FRET signal was measured on an EnVision plate reader (PerkinElmer) with excitation at 340 nm and dual emission readout at 495 and 520 nm with the delay time of 100 micro second and the integration time of 200 micro second. The ratio of emission signal at 520 and at 495 was used to calculate EC50 using GraphPad Prism (GraphPad Software). In every batch of compound screening, T3 (L-3,3′,5-Triiodothyronine sodium salt, >95%) (Calbiochem) was used as reference compound. The EC50 of T3 measured were within 3-fold of the reference value provided by the assay kit manufacturer (ThermoFisher Scientific). The Z′ factors measured in every batch of screening using T3 as high percent effect (HPE) control and 0.5% DMSO as zero percent effect (ZPE) control were in the range of 0.5 to 0.8. Compounds' THR-beta selectivity values are derived from T3-selectivity normalized data.
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay for Thyroid Hormone Receptor Agonist Screening LanthaScreen TR-FRET Thyroid Receptor alpha Coactivator Assay kit (ThermoFisher) and LanthaScreen TR-FRET Thyroid Receptor beta Coactivator Assay kit (ThermoFisher) were used for agonist compound screening. Compounds in DMSO were diluted using ECHO Liquid Handler (Labcyte Inc.) into 384 plates in 10-point 3-fold series in duplicate (5 micro M final top concentration). Buffer C (ThermoFisher) was added to each well before the 4× mixture of fluorescein-SCR2-2 coactivator (200 nM final concentration), Terbium-labeled anti-GST antibody (2 nM final concentration), and TR alpha-LBD (0.4 nM final concentration) or TR beta-LBD (1.0 nM final concentration) was added. After 2 hour incubation at room temperature in dark, the TR-FRET signal was measured on an EnVision plate reader (PerkinElmer) with excitation at 340 nm and dual emission readout at 495 and 520 nm with the delay time of 100 micro second and the integration time of 200 micro second. The ratio of emission signal at 520 and at 495 was used to calculate EC50 using GraphPad Prism (GraphPad Software). In every batch of compound screening, T3 (L-3,3′,5-Triiodothyronine sodium salt, >95%) (Calbiochem) was used as reference compound. The EC50 of T3 measured were within 3-fold of the reference value provided by the assay kit manufacturer (ThermoFisher Scientific). The Z′ factors measured in every batch of screening using T3 as high percent effect (HPE) control and 0.5% DMSO as zero percent effect (ZPE) control were in the range of 0.5 to 0.8. Compounds' THR-beta selectivity values are derived from T3-selectivity normalized data.
- TBD Briefly, 10× stock solutions of MK2 (PV3317, from Life Technologies), 1.13X ATP (AS001A), and Sox conjugated peptide substrate, S/T3-Sox, (KZN1031) were prepared in 1× kinase reaction buffer consisting of 20 mM Tris, pH 7.5, 5 mM MgCl2, 1 mM EGTA, 5 mM β-glycerophosphate, 5% glycerol (10× stock, KB001A) and 0.2 mM DTT (DS001A). Enzyme solution (5 μL) was added to each of DMSO (5 μL) or serially diluted test compounds prepared in DMSO in a Corning (#3574) 384-well, white, non-binding surface microtiter plate (Corning, NY). Kinase reactions were started with the addition of 45 μL of the ATP-peptide substrate S/T3-Sox mix and monitored every 71 seconds for 120 minutes at λex360/λem485 in a Synergy H4 plate reader from BioTek (Winooski, VT) at room temperature.
- THR/RXR Heterodimer Assay for Thyroid Hormone Receptor Agonist Screening Test compounds were prepared as 10 mM DMSO stock solutions. The stock solution (45 uL) was transferred to a 384-well assay plate, and 3-fold, 10-point dilutions were performed by transferring 15 μL of the compound solution into 30 μL DMSO using TECAN (EVO200) liquid handler. The compound solutions (200 nL, serially diluted) and the positive control triiodothyronine (T3) (100 nL) were transferred to an assay plate using ECHO550. Next, H6-THR-α (150.64 uM, 10 μL) or H6-THR-β (32.57 uM, 10 μL) in binding buffer (50 mM HEPES, pH 7.0, 1 mM DTT, 0.05% NP40, 0.2 mg/mL BSA) was mixed with retinoid X receptor alpha (RxRα) (146.76 uM, 10 μL) in binding buffer, and transferred to the 384-well assay plate containing T3 or compound solution. After incubation at 37° C. for 30 min, biotin-GRIP1 peptide (3262.1 uM, 10 μL) in binding buffer and 5% DMSO was added to the 384-well assay plate and incubated at 37° C. for 30 min. A solution (10 μL) containing europium-conjugated anti-hexa(His) antibody (0.625 uM) and APC-conjugated streptavidin (1.18 uM) in buffer (50 mM Tris, pH 7.4, 100 mM NaCl, and 0.2 mg/mL BSA) was then added to the 384-well assay plate and incubated at 25° C. for 60 min. The assay plate was read using Envision (PerkinElmer), using T3 as the positive control for both THR-β/RXR-α and THR-α/RXR-α activity. DMSO was used as the negative control. Compound activity for the THR-β/RXR-α and THR-α/RXR-α assays were normalized to T3 activity for each assay run. THR-β selectivity was calculated by dividing the normalized THR-β/RXR-α compound activity by the normalized THR-α/RXR-α compound activity.
- Recruitment Assay Thirty microliters of H6-TRβ (50 nM) in 50 mM Hepes, pH 7.0, 1 mM DTT, 0.05% NP40 and 0.2 mg/ml BSA (Binding Buffer) was mixed with an equal volume of EE-RxRα (50 nM) in Binding Buffer. Six microliters of T3 (0-14.8 uM) or test compound (0-1.2 mM) in DMSO was then added and the solution incubated at 37° C. for 30 min. Thirty microliters of biotin-GRIP peptide (Biotin-Aca-HGTSLKEKHKILHRLLQDSSSPVDL-CONH2) (100 nM) in 30 ul of Binding Buffer plus 5% DMSO was then added and the solution incubated at 37° C. for 30 min. Thirty microliters of solution containing 12 nM europium-conjugated anti-hexa His antibody and 160 nM APC-conjugated streptavidin in 50 mM Tris, pH 7.4, 100 mM NaCl and 0.2 mg/ml BSA was added and the solution incubated at 4° C. for over night. An aliquot (35 ul/sample) was transferred to 384-well black microtiter plates. The HTRF signal was read on the Victor 5 reader (PerkinElmer Life and Analytical Sciences).
- THR Reporter Gene Method Huh7 cells were cultured in a DMEM medium supplemented with 10% FBS. The cells were inoculated into a 10 cm cell culture dish, proliferated to about 90% confluency, and co-transfected by using a liposome (Lipofectamine 2000) with human THRa eukaryotic expression plasmid or human THRβ eukaryotic expression plasmid, as well as reporter gene plasmid PGL4.26-DR4-Luc containing THR response sequence driver. The operation steps were carried out according to the instructions of Lipofectamine 2000. The next day after transfection, the cells were inoculated with a phenol red-free DMEM (supplemented with 5% activated carbon-treated FBS) into a 96-well cell culture plate at a seeding density of 20,000 cells per well and a volume of 135 μL per well. The cells adhered to wall 6 hours after the inoculation. Compounds dissolved in DMSO were diluted in phenol red-free DMEM (supplemented with 5% activated carbon-treated FBS) to be 20 to 10 times of the final concentration, and added to the cell wells at 15 μL per well, that is, the compounds were diluted 10 times again to reach the final concentration. Triiodothyronine T3 (100 nM) was set as a positive control, and 0.5% DMSO was set as a blank control. After the compounds were added, the cells were cultured at 37 C. in a 5% CO2 incubator overnight (16 hours). After incubation, the culture medium was discarded, and each well was added with 35 μL of serum-free and phenol red-free DMEM and 35 μL of Steady-Glo, shaked for 10 minutes in the dark, and the chemiluminescence value of the sample was detected. The agonistic activity of the compound was calculated as follows: % effect=(compound−blank control)/(positive control−blank control) 100%. The EC50 of the compound was obtained by fitting the agonistic activity of the compound and the logarithmic value of the compound concentration with GraphPad Prism. The lower the EC50 value, the better the activity.
 US9878991, Compound T3 BDBM236160 US9365563, T3
US9878991, Compound T3 BDBM236160 US9365563, T3 BDBM50592246 T3-CLK
BDBM50592246 T3-CLK BDBM511489 US11059792, Compound T3
BDBM511489 US11059792, Compound T3 BDBM546519 US11292781, Compound T3
BDBM546519 US11292781, Compound T3 [125I]T3 US10544075, Compound T3 CHEMBL1544 triothyrone Triiodothyronine Triiodothyronine (T3) 3,5,3'-triiodo-L-thyronine (T3) (2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid BDBM18860 liothyronine
[125I]T3 US10544075, Compound T3 CHEMBL1544 triothyrone Triiodothyronine Triiodothyronine (T3) 3,5,3'-triiodo-L-thyronine (T3) (2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid BDBM18860 liothyronine US9303033, T3, Table 13A, Compound 2 BDBM218913 US9303033, H7, Table 22A, Compound 2
US9303033, T3, Table 13A, Compound 2 BDBM218913 US9303033, H7, Table 22A, Compound 2 CHEMBL105983 BDBM50130595 2-Amino-3-[4-(4-hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-phenyl]-propionic acid Dimethyl-isopropyl-T3 (DIMIT)
CHEMBL105983 BDBM50130595 2-Amino-3-[4-(4-hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-phenyl]-propionic acid Dimethyl-isopropyl-T3 (DIMIT) US10087178, Example T3 BDBM288135 (1S,2S)-N-(2,4- difluorobenzyl)-9'-hydroxy-2- (methoxymethyl)-2'-methyl- 1',8'-dioxo-1',2',3',8'- tetrahydrospiro[cyclopropane- 1,4'-pyrido[1,2-a]pyrazine]- 7'-carboxamide hydrochloride
US10087178, Example T3 BDBM288135 (1S,2S)-N-(2,4- difluorobenzyl)-9'-hydroxy-2- (methoxymethyl)-2'-methyl- 1',8'-dioxo-1',2',3',8'- tetrahydrospiro[cyclopropane- 1,4'-pyrido[1,2-a]pyrazine]- 7'-carboxamide hydrochloride