- abl mutant (m351t)
- abl mutant (t315i)
- abl mutant (t315n)
- abl mutant (y253f)
- braf mutant v600e
- chitinase mutant (m243a)
- ksp mutant (a356t)
- noxab mutant (e74f)
- puma mutant (m144i)
- src mutant (s345c)
- src mutant (t338m)
- cyclooxygenase-2 mutant (y355f)
- hcv ns5b mutant(s283t)
- hyhel-10 mutant (aeaf)
- hyhel-10 mutant (etkf)
- hyhel-10 mutant (hy58a)
- hyhel-10 mutant (hy58f)
- hyhel-10 mutant (sfsf)
- hyhel-5 mutant(e50d)
- hyhel-5 mutant(e50q)
- mtor/ src mutant (t338i)
- ret kinase mutant (g810r)
- src mutant (s345c/t338m)
- urokinase (upa) mutant (s190a)
- aldose reductase (alr2) mutant (l300p)
- bira bifunctional protein mutant (v214a)
- bira bifunctional protein mutant (v219a)
- bira bifunctional protein mutant (w223a)
- carbonic anhydrase ii mutant (f130v)
- dihydrofolate reductase (dhfr) mutant (f98y)
- dihydrofolate reductase (dhfr) mutant (i100l)
- dihydroorotate dehydrogenase (dhodh) mutant (h185a)
- dihydroorotate dehydrogenase (dhodh) mutant (r265a)
- dna-pk/ src mutant (t338i)
- hcv ns5b polymerase mutant (p495a)
- hcv ns5b polymerase mutant (r501e)
- hiv-1 protease mutant (a71v)
- hiv-1 protease mutant (d30n)
- hiv-1 protease mutant (g73s)
- hiv-1 protease mutant (i47l)
- hiv-1 protease mutant (i50l)
- hiv-1 protease mutant (i50v)
- hiv-1 protease mutant (i84v)
- hiv-1 protease mutant (l23i)
- hiv-1 protease mutant (l23v)
- hiv-1 protease mutant (l76m)
- hiv-1 protease mutant (v32i)
- hiv-1 protease mutant (v82a)
- hiv-1 protease mutant (v82i)
- hiv-1 protease triple mutant
- hyhel-10 fv mutant (hd32a)
- hyhel-10 fv mutant (hd32e)
- hyhel-10 fv mutant (hd32n)
- isocitrate dehydrogenase 2 mutant (r172s)
- p110-delta/ src mutant (t338i)
- pi3k-alpha/ src mutant (t338i)
- transcriptional regulator ttgr mutant r176g
- tyrosine kinase flt3 mutant (d835h)
- tyrosine kinase flt3 mutant (d835y)
- b-raf protein kinase mutant (v599e)
- cytochrome p450 51(cyp51) mutant (f78l)
- egf-r tyrosine kinase mutant (g719c)
- egf-r tyrosine kinase mutant (g719s)
- egf-r tyrosine kinase mutant (l858r)
- egf-r tyrosine kinase mutant (t790m)
- enoyl-acp reductase (fabi) mutant (f203l)
- estrogen-related receptor, alpha mutant (c325s)
- hiv-1 protease mutant (d30n/a71v)
- hiv-1 protease mutant (d30n/m36i)
- hiv-1 protease mutant (l10i/l90m)
- hiv-1 protease mutant (m36i/a71v)
- hiv-1 protease mutant (m46i/i54v)
- hiv-1 protease mutant (v82a/i84v)
- hiv-1 protease mutant (v82f/i84v)
- hiv-1 protease mutant k-60c
- hiv-1 protease mutant v-18c
- hiv-1 protease mutant, a-1
- hiv-1 protease mutant, mdr-hm
- hiv-1 protease mutant, mdr-qm
- hiv-1 protease mutant, nam-10
- hiv-1 reverse transcriptase mutant (k103n)
- hiv-1 reverse transcriptase mutant (l100i)
- hiv-1 reverse transcriptase mutant (p236l)
- hiv-1 reverse transcriptase mutant (v106a)
- hiv-1 reverse transcriptase mutant (v179d)
- hiv-1 reverse transcriptase mutant (y181c)
- hiv-1 reverse transcriptase mutant (y188l)
- leucyl-trna synthetase k510e mutant (k510e)
- map kinase p38 alpha mutant (g110a)
- map kinase p38 alpha mutant (g110d)
- nitric oxide synthase mutant (enos y477a)
- nitric oxide synthase mutant (nnos h341l)
- nitric oxide synthase, brain mutant (d597n)
- nitric oxide synthase, endothelial mutant (n368d)
- pfdhfr-ts double mutant (c59r+s108n)
- pretherapy isolated ae protease mutant (v82f)
- tyrosine kinase c-met mutant (d1228h)
- 20-alpha-hydroxysteroid dehydrogenase (akr1c1) mutant (h222s)
- 20-alpha-hydroxysteroid dehydrogenase (akr1c1) mutant (l306a)
- 20-alpha-hydroxysteroid dehydrogenase (akr1c1) mutant (l54v)
- camp-dependent protein kinase (pka) mutant (e127d)
- cyclin-dependent kinase 2 (cdk2) mutant (f82h)
- cyclin-dependent kinase 2 (cdk2) mutant (k89t)
- cystic fibrosis transmembrane conductance regulator mutant g550e
- hiv-1 protease b subtype mutant (v82f)
- hiv-1 protease drug-resistant mutant 1
- hiv-1 protease mutant (a71v/v82t/i84v)
- hiv-1 reverse transcriptase mutant (103n/181c)
- hiv-1 reverse transcriptase mutant (l100i/k103n)
- protein-tyrosine phosphatase 1b (ptp1b) mutant (g117e)
- protein-tyrosine phosphatase 1b (ptp1b) mutant (l119v)
- protein-tyrosine phosphatase 1b (ptp1b) mutant (m114v)
- src homology 2 domain mutant (sh2-58.6)
- tyrosine kinase c-kit mutant (v559d/t670i)
- camp-dependent protein kinase (pka) mutant (l49i/t183a)
- camp-dependent protein kinase (pka) mutant (q181k/t183a)
- camp-dependent protein kinase (pka) mutant pkar1 (3)
- dihydrofolate reductase-thymidylate synthase (dhfr-ts) mutant kicb1
- hiv-1 protease a subtype mutant (v82f/i84v)
- hiv-1 protease b subtype mutant (v82f/i84v)
- hiv-1 protease c subtype mutant (v82f/i84v)
- hiv-1 protease mutant v6(46/54/84)
- hiv-1 reverse transcriptase rnase h mutant (y181c)
- hiv-1 reverse transcriptase rnase h mutant (y188l)
- mitogen-activated protein kinase 9 (jnk2) mutant (c162s)
- mitogen-activated protein kinase 9 (jnk2) mutant (r127a)
- monoamine oxidase type b (mao-b) mutant (i199f)
- phosphodiesterase type 5 (pde5a) h-loop deletion mutant
- protein-tyrosine phosphatase 1b (ptp1b) mutant (v113l/m114v)
- proto-oncogene tyrosine-protein kinase abl1 mutant f359v
- proto-oncogene tyrosine-protein kinase abl1 mutant q252h
- src homology 2 domain mutant (t40v/c45a/k60l)
- tyrosine kinase csf1-r mutant (tie2 kid chimera)
- tyrosyl-dna phosphodiesterase 2 mutant 9m(mtdp2 9m)
- 3-phosphoinositide-dependent protein kinase 1 (pdk1) mutant (e166d)
- 3-phosphoinositide-dependent protein kinase 1 (pdk1) mutant (l159m)
- 3-phosphoinositide-dependent protein kinase 1 (pdk1) mutant (t222a)
- 3-phosphoinositide-dependent protein kinase 1 (pdk1) mutant (v143t)
- camp-dependent protein kinase (pka) mutant (v123m/q181k/t183a)
- f420-dependent glucose-6-phosphate dehydrogenase mutant (fgd e109q)
- f420-dependent glucose-6-phosphate dehydrogenase mutant (fgd h40a)
- hiv-1 protease mutant (l10i,l19q,k20r,e35d,m36i, ..)
- hiv-1 protease mutant (m46i,l63p,a71v,v82f,i84v)
- hiv-1 reverse transcriptase rnase h mutant (v106a/y181c)
- phosphoinositide 3-kinase (pi3k), alpha mutant (h1047r) chain a
- 3-phosphoinositide-dependent protein kinase 1 (pdk1) mutant (v143t/t222a)
- cyclin-dependent kinase 2 (cdk2) mutant (f82h/l83v/h84d/k89t)
- gabaa receptor mutant, alpha-1 (a160c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (d97c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (f99c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (g157c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (s204c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (t162c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1 (y209c), beta-2, gamma-2
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (d56c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (f77c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (l140c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (r132c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (r144c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (r185c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (r194c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (t126c)
- gabaa receptor mutant, alpha-1, beta-2, gamma-2 (t142c)
- hiv-1 protease mutant m1 (l10i, g48v, i54v, l63p, v82a)
- phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha isoform mutant
- camp-dependent protein kinase (pka) mutant (l49i/v123m/e127d/q181k/t183a)
- hiv-1 reverse transcriptase rnase h mutant (d67n/k70r/t215f/k219q)
- post-therapy hiv-1 crf_01 a/e protease f82v back mutant
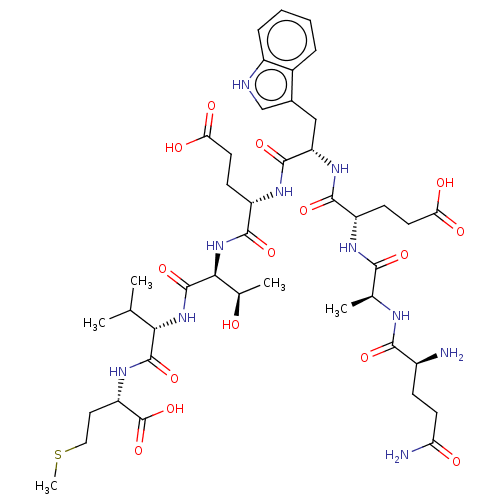 PTHRct-8 mutant (QAEWETVM) QAEWETVM BDBM228841
PTHRct-8 mutant (QAEWETVM) QAEWETVM BDBM228841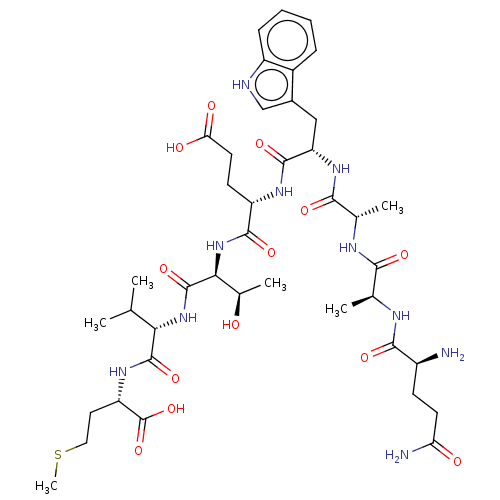 QAAWETVM PTHRct-8 mutant (QAAWETVM) BDBM228843
QAAWETVM PTHRct-8 mutant (QAAWETVM) BDBM228843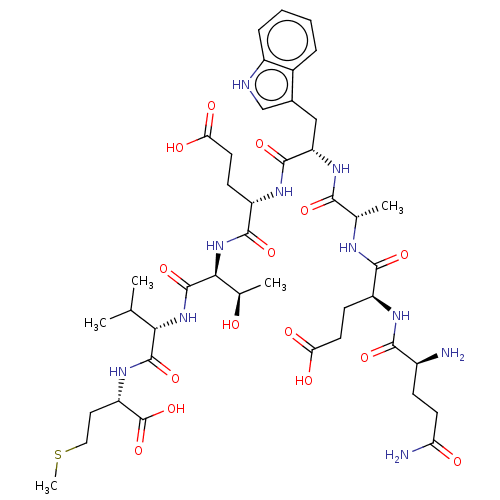 QEAWETVM PTHRct-8 mutant (QEAWETVM) BDBM228842
QEAWETVM PTHRct-8 mutant (QEAWETVM) BDBM228842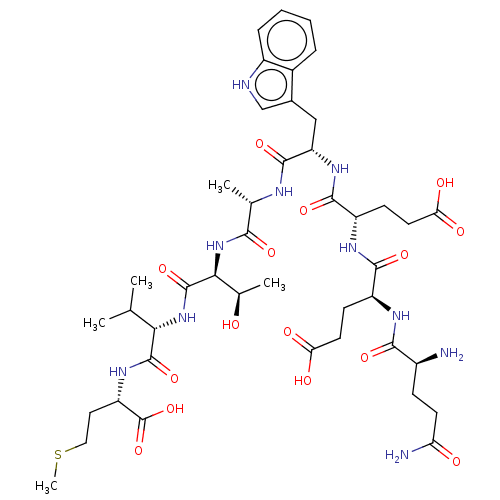 QEEWATVM PTHRct-8 mutant (QEEWATVM) BDBM228844
QEEWATVM PTHRct-8 mutant (QEEWATVM) BDBM228844
- Haque, R; Maity, D Small molecule-based fluorescent probes for the detection of α-Synuclein aggregation states. Bioorg Med Chem Lett 86: (2023)
- Oliveri, V Toward the discovery and development of effective modulators of α-synuclein amyloid aggregation. Eur J Med Chem 167: 10-36 (2019)
- Zeng, Q; Liu, S; Cui, M Structure-Activity Relationships of Cyano-substituted Indole Derivatives as Ligands for α-Synuclein Aggregates. ACS Med Chem Lett 14: 1467-1471 (2023)
- Han, F; Jiang, B; Lü, MH; Wang, ZP; Liu, W; Zhang, YX; Xu, J Hybrids of polyphenolic acids and xanthone, the potential preventive and therapeutic effects on PD: Design, synthesis, in vitro anti-aggregation of α-synuclein, and disaggregation against the existed α-synuclein oligomer and fibril. Bioorg Med Chem 66: (2022)
- Liu, W; Zhang, W; Xing, LZ; Zhao, YD; Xu, J; Li, RJ; Zhang, YX 4-Arylidene curcumin derivatives in vitro inhibit α-Synuclein aggregation and disaggregate the preformed fibril. Bioorg Med Chem 96: (2023)
- Liu, H; Chen, L; Zhou, F; Zhang, YX; Xu, J; Xu, M; Bai, SP Anti-oligomerization sheet molecules: Design, synthesis and evaluation of inhibitory activities against α-synuclein aggregation. Bioorg Med Chem 27: 3089-3096 (2019)
- Elbatrawy, AA; Ademoye, TA; Alnakhala, H; Tripathi, A; Zami, A; Ostafe, R; Dettmer, U; Fortin, JS Discovery of small molecule benzothiazole and indole derivatives tackling tau 2N4R and α-synuclein fibrils. Bioorg Med Chem 100:
- Qiu, C; Wei, R; Bian, J; Lin, X; Bai, T; He, J; Guo, X; Chu, Y Novel 4-triazole phenyl amide (4-TPA) molecules: Potent promoters of α-synuclein fibril disassembly. Eur J Med Chem 273:
- Xing, LZ; Zhang, W; Zhao, YD; Xu, J; Zhang, YX Pyrazolamide derivatives inhibit α-Synuclein aggregation, disaggregate preformed fibers, and reduce inclusion formation in neuron cells. Eur J Med Chem 268:
- AlNajjar, YT; Gabr, M; ElHady, AK; Salah, M; Wilms, G; Abadi, AH; Becker, W; Abdel-Halim, M; Engel, M Discovery of novel 6-hydroxybenzothiazole urea derivatives as dual Dyrk1A/α-synuclein aggregation inhibitors with neuroprotective effects. Eur J Med Chem 227: (2022)
- Chen, L; Huang, GL; Lü, MH; Zhang, YX; Xu, J; Bai, SP Amide derivatives of Gallic acid: Design, synthesis and evaluation of inhibitory activities against in vitro α-synuclein aggregation. Bioorg Med Chem 28: (2020)
- Zhao, YD; Zhang, W; Xing, LZ; Xu, J; Shi, WM; Zhang, YX In vitro inhibition of α-Synuclein aggregation and disaggregation of preformed fibers by polyphenol hybrids with 2-conjugated benzothiazole. Bioorg Med Chem Lett 105:
- Tu, Z; Kotzbauer, PT; Yue, X; Dhavale, DD Alpha-synuclein ligands US Patent US12012394 (2024)
- Chen, YF; Bian, J; Zhang, P; Bu, LL; Shen, Y; Yu, WB; Lu, XH; Lin, X; Ye, DY; Wang, J; Chu, Y Design, synthesis and identification of N, N-dibenzylcinnamamide (DBC) derivatives as novel ligands for α-synuclein fibrils by SPR evaluation system. Bioorg Med Chem 28: (2020)
- Zhang, W; Liu, W; Zhao, YD; Xing, LZ; Xu, J; Li, RJ; Zhang, YX The potential of Rhein's aromatic amines for Parkinson's disease prevention and treatment: α-Synuclein aggregation inhibition and disaggregation of preformed fibers. Bioorg Med Chem Lett 97:
- Jiang, B; Han, F; Lü, MH; Wang, ZP; Liu, W; Zhang, YX; Xu, J; Li, RJ Bis-chalcone polyphenols with potential preventive and therapeutic effects on PD: Design, synthesis and in vitro disaggregation activity against α-synuclein oligomers and fibrils. Eur J Med Chem 239: (2022)
- Maestri, V; Tarozzi, A; Simoni, E; Cilia, A; Poggesi, E; Naldi, M; Nicolini, B; Pruccoli, L; Rosini, M; Minarini, A Quinazoline based α Eur J Med Chem 136: 259-269 (2017)
- Ahsan, N; Siddique, IA; Gupta, S; Surolia, A A routinely used protein staining dye acts as an inhibitor of wild type and mutant alpha-synuclein aggregation and modulator of neurotoxicity. Eur J Med Chem 143: 1174-1184 (2018)
- Hammock, BD; Hwang, SH; Hashimoto, K; Ren, Q Methods of inhibiting formation of alpha synuclein aggregates US Patent US12251379 (2025)
- Yu, L; Cui, J; Padakanti, PK; Engel, L; Bagchi, DP; Kotzbauer, PT; Tu, Z Synthesis and in vitro evaluation ofa-synuclein ligands. Bioorg Med Chem 20: 4625-34 (2012)
- Jang, M; Tempest, P; Lin, Y Compounds for degrading alpha-synuclein aggregates and uses thereof US Patent US12377152 (2025)
- Tong, Y; Zhu, W; Chen, J; Wen, T; Xu, F; Pang, J Discovery of Small-Molecule Degraders for Alpha-Synuclein Aggregates. J Med Chem 66: 7926-7942 (2023)
- Anselmi, M; Baiula, M; Spampinato, S; Artali, R; He, T; Gentilucci, L Design and Pharmacological Characterization of α J Med Chem 66: 5021-5040 (2023)
- Flores-Bocanegra, L; González-Andrade, M; Bye, R; Linares, E; Mata, R α-Glucosidase Inhibitors from Salvia circinata. J Nat Prod 80: 1584-1593 (2017)
- Janssen, FJ; Baggelaar, MP; Hummel, JJ; Overkleeft, HS; Cravatt, BF; Boger, DL; van der Stelt, M Comprehensive Analysis of Structure-Activity Relationships of α-Ketoheterocycles as sn-1-Diacylglycerol Lipase α Inhibitors. J Med Chem 58: 9742-53 (2015)
- Citarella, A; Cavinato, M; Rosini, E; Shehi, H; Ballabio, F; Camilloni, C; Fasano, V; Silvani, A; Passarella, D; Pollegioni, L; Nardini, M Nicotinic Acid Derivatives As Novel Noncompetitive α-Amylase and α-Glucosidase Inhibitors for Type 2 Diabetes Treatment. ACS Med Chem Lett 15: 1474-1481
- Bian, J; Liu, YQ; He, J; Lin, X; Qiu, CY; Yu, WB; Shen, Y; Zhu, ZY; Ye, DY; Wang, J; Chu, Y Discovery of styrylaniline derivatives as novel alpha-synuclein aggregates ligands. Eur J Med Chem 226: (2021)
- Tong, Y; Zhu, W; Chen, J; Zhang, W; Xu, F; Pang, J Targeted Degradation of Alpha-Synuclein by Autophagosome-Anchoring Chimera Peptides. J Med Chem 66: 12614-12628 (2023)
- Lefin, R; van der Walt, MM; Milne, PJ; Terre'Blanche, G Imidazo[1,2-α]pyridines possess adenosine A Bioorg Med Chem Lett 27: 3963-3967 (2017)
- McMullin, DR; Nsiama, TK; Miller, JD Isochromans and α-pyrones from Penicillium corylophilum. J Nat Prod 77: 206-12 (2014)
- Meck, C; D'Erasmo, MP; Hirsch, DR; Murelli, RP The biology and synthesis of α-hydroxytropolones. Medchemcomm 5: 842-852 (2014)
- Abás, S; Rodríguez-Arévalo, S; Bagán, A; Griñán-Ferré, C; Vasilopoulou, F; Brocos-Mosquera, I; Muguruza, C; Pérez, B; Molins, E; Luque, FJ; Pérez-Lozano, P; de Jonghe, S; Daelemans, D; Naesens, L; Brea, J; Loza, MI; Hernández-Hernández, E; García-Sevilla, JA; García-Fuster, MJ; Radan, M; Djikic, T; Nikolic, K; Pallàs, M; Callado, LF; Escolano, C Bicyclic α-Iminophosphonates as High Affinity Imidazoline I J Med Chem 63: 3610-3633 (2020)
- Lippa, RA; Barrett, J; Pal, S; Rowedder, JE; Murphy, JA; Barrett, TN Discovery of the first potent and selective α Eur J Med Chem 208: (2020)
- Rísquez-Cuadro, R; Matsumoto, R; Ortega-Caballero, F; Nanba, E; Higaki, K; García Fernández, JM; Ortiz Mellet, C Pharmacological Chaperones for the Treatment of α-Mannosidosis. J Med Chem 62: 5832-5843 (2019)
- Gehrke, NR; Feng, D; Ayub Ali, M; Maalouf, MA; Holstein, SA; Wiemer, DF α-Amino bisphosphonate triazoles serve as GGDPS inhibitors. Bioorg Med Chem Lett 102:
- Merlino, F; Zhou, Y; Cai, M; Carotenuto, A; Yousif, AM; Brancaccio, D; Di Maro, S; Zappavigna, S; Limatola, A; Novellino, E; Grieco, P; Hruby, VJ Development of Macrocyclic Peptidomimetics Containing Constrained α,α-Dialkylated Amino Acids with Potent and Selective Activity at Human Melanocortin Receptors. J Med Chem 61: 4263-4269 (2018)
- Luo, S; Zhao, L; Peng, H; Peng, Z; Wang, G Novel carbazole-oxadiazole derivatives as anti-α-glucosidase and anti-α-amylase agents: Design, synthesis, molecular docking, and biological evaluation. Eur J Med Chem 275:
- Barrett, TN; Taylor, JA; Barker, D; Procopiou, PA; Thompson, JDF; Barrett, J; Le, J; Lynn, SM; Pogany, P; Pratley, C; Pritchard, JM; Roper, JA; Rowedder, JE; Slack, RJ; Vitulli, G; Macdonald, SJF; Kerr, WJ Profile of a Highly Selective Quaternized Pyrrolidine Betaine α J Med Chem 62: 7543-7556 (2019)
- Nardelli, F; Paissoni, C; Quilici, G; Gori, A; Traversari, C; Valentinis, B; Sacchi, A; Corti, A; Curnis, F; Ghitti, M; Musco, G Succinimide-Based Conjugates Improve IsoDGR Cyclopeptide Affinity to α J Med Chem 61: 7474-7485 (2018)
- Rangel-Grimaldo, M; Macías-Rubalcava, ML; González-Andrade, M; Raja, H; Figueroa, M; Mata, R α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from J Nat Prod 83: 675-683 (2020)
- He, HW; Peng, H; Wang, T; Wang, C; Yuan, JL; Chen, T; He, J; Tan, X α-(Substituted-phenoxyacetoxy)-α-heterocyclylmethylphosphonates: synthesis, herbicidal activity, inhibition on pyruvate dehydrogenase complex (PDHc), and application as postemergent herbicide against broadleaf weeds. J Agric Food Chem 61: 2479-88 (2013)
- Outeiro, TF; Kontopoulos, E; Altmann, SM; Kufareva, I; Strathearn, KE; Amore, AM; Volk, CB; Maxwell, MM; Rochet, JC; McLean, PJ; Young, AB; Abagyan, R; Feany, MB; Hyman, BT; Kazantsev, AG Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science 317: 516-9 (2007)
- Santos, CMM; Freitas, M; Fernandes, E A comprehensive review on xanthone derivatives as α-glucosidase inhibitors. Eur J Med Chem 157: 1460-1479 (2018)
- Osella, MI; Salazar, MO; Gamarra, MD; Moreno, DM; Lambertucci, F; Frances, DE; Furlan, RLE Arylsulfonyl histamine derivatives as powerful and selective α-glucosidase inhibitors. RSC Med Chem 11: 518-527 (2020)
- Jacobsson, E; Peigneur, S; Andersson, HS; Laborde, Q; Strand, M; Tytgat, J; Göransson, U Functional Characterization of the Nemertide α Family of Peptide Toxins. J Nat Prod 84: 2121-2128 (2021)
- Pflieger, M; Hamacher, A; Öz, T; Horstick-Muche, N; Boesen, B; Schrenk, C; Kassack, MU; Kurz, T Novel α,β-unsaturated hydroxamic acid derivatives overcome cisplatin resistance. Bioorg Med Chem 27: (2019)
- Lynagh, T; Kiontke, S; Meyhoff-Madsen, M; Gless, BH; Johannesen, J; Kattelmann, S; Christiansen, A; Dufva, M; Laustsen, AH; Devkota, K; Olsen, CA; Kümmel, D; Pless, SA; Lohse, B Peptide Inhibitors of the α-Cobratoxin-Nicotinic Acetylcholine Receptor Interaction. J Med Chem 63: 13709-13718 (2020)
- Kaur, B; Mishra, S; Kaur, R; Kalotra, S; Singh, P Rationally designed TNF-α inhibitors: Identification of promising cytotoxic agents. Bioorg Med Chem Lett 41: (2021)
- He, H; Liu, Z; Wang, W; Jiang, X Synthesis and cytotoxic evaluation of halogenated α-exo-methylene-lactones. Bioorg Med Chem 28: (2020)
- Dhameja, M; Gupta, P Synthetic heterocyclic candidates as promising α-glucosidase inhibitors: An overview. Eur J Med Chem 176: 343-377 (2019)
- Khamees Thabet, H; Ragab, A; Imran, M; Helal, MH; Ibrahim Alaqel, S; Alshehri, A; Ash Mohd, A; Rakan Alshammari, M; S Abusaif, M; A Ammar, Y Discovery of new anti-diabetic potential agents based on paracetamol incorporating sulfa-drugs: Design, synthesis, α-amylase, and α-glucosidase inhibitors with molecular docking simulation. Eur J Med Chem 275:
- Hameed, S; Kanwal, na; Seraj, F; Rafique, R; Chigurupati, S; Wadood, A; Rehman, AU; Venugopal, V; Salar, U; Taha, M; Khan, KM Synthesis of benzotriazoles derivatives and their dual potential as α-amylase and α-glucosidase inhibitors in vitro: Structure-activity relationship, molecular docking, and kinetic studies. Eur J Med Chem 183: (2019)
- Handzlik, J; Bajda, M; Zygmunt, M; Maciąg, D; Dybała, M; Bednarski, M; Filipek, B; Malawska, B; Kieć-Kononowicz, K Antiarrhythmic properties of phenylpiperazine derivatives of phenytoin with α₁-adrenoceptor affinities. Bioorg Med Chem 20: 2290-303 (2012)
- Meibom, D; Meyer, J; von Buehler, CJ; Collins, KD; Maassen, S; Gericke, KM; Hüser, J; Mittendorf, J; Ortega Hernandez, N; Schamberger, J; Stampfuss, J; Straub, A; Torge, A; Witowski, N; Wunder, F BAY-6096: A Potent, Selective, and Highly Water-Soluble Adrenergic α J Med Chem 66: 4659-4670 (2023)
- Liu, J; Cheng, X; Tian, X; Guan, D; Ao, J; Wu, Z; Huang, W; Le, Z Design and synthesis of novel dual-cyclic RGD peptides for α Bioorg Med Chem Lett 29: 896-900 (2019)
- Wang, X; Edwards, RL; Ball, H; Johnson, C; Haymond, A; Girma, M; Manikkam, M; Brothers, RC; McKay, KT; Arnett, SD; Osbourn, DM; Alvarez, S; Boshoff, HI; Meyers, MJ; Couch, RD; Odom John, AR; Dowd, CS MEPicides: α,β-Unsaturated Fosmidomycin Analogues as DXR Inhibitors against Malaria. J Med Chem 61: 8847-8858 (2018)
- Nencetti, S; La Motta, C; Rossello, A; Sartini, S; Nuti, E; Ciccone, L; Orlandini, E N-(Aroyl)-N-(arylmethyloxy)-α-alanines: Selective inhibitors of aldose reductase. Bioorg Med Chem 25: 3068-3076 (2017)
- Liu, X; Tao, J; Zhang, S; Lan, W; Wang, C; Ji, Y; Cao, C Selective Blockade of Neuronal BK (α + β4) Channels Preventing Epileptic Seizure. J Med Chem 63: 216-230 (2020)
- Chen, Y; Jia, L; Zhu, G; Wang, W; Geng, M; Lu, H; Zhang, Y; Zhou, M; Zhang, F; Cheng, X Sortase A-mediated cyclization of novel polycyclic RGD peptides for α Bioorg Med Chem Lett 73: (2022)
- Kongphet, M; Hang, HTX; Ngo, TT; Le, TK; Chavasiri, W Structural modification of tanshinone IIA and their α-glucosidase inhibitory activity. Bioorg Med Chem Lett 105:
- Sari, O; Roy, V; Métifiot, M; Marchand, C; Pommier, Y; Bourg, S; Bonnet, P; Schinazi, RF; Agrofoglio, LA Synthesis of dihydropyrimidine α,γ-diketobutanoic acid derivatives targeting HIV integrase. Eur J Med Chem 104: 127-38 (2015)
- Deng, H; Li, W Therapeutic potential of targeting α/β-Hydrolase domain-containing 6 (ABHD6). Eur J Med Chem 198: (2020)
- Jiang, ZY; Feng, JE; Duan, LK; Liu, CJ; Li, XF; Huang, CQ; Shi, SL; Wang, RR; Zuo, AX; He, HP Tigliane Diterpenoids with Larvicidal, Antifungal, and α-Glucosidase Inhibitory Activities from J Nat Prod 85: 405-414 (2022)
- Loesche, A; Wiemann, J; Al Halabi, Z; Karasch, J; Sippl, W; Csuk, R Unexpected AChE inhibitory activity of (2E)α,β-unsaturated fatty acids. Bioorg Med Chem Lett 28: 3315-3319 (2018)
- Kubo, M; Yamamoto, K; Itoh, T Design and synthesis of selective CYP1B1 inhibitor via dearomatization of α-naphthoflavone. Bioorg Med Chem 27: 285-304 (2019)
- Sun, W; Wu, Y; Zheng, M; Yang, Y; Liu, Y; Wu, C; Zhou, Y; Zhang, Y; Chen, L; Li, H Discovery of an Orally Active Small-Molecule Tumor Necrosis Factor-α Inhibitor. J Med Chem 63: 8146-8156 (2020)
- Allison, M; Davie, RL; Mogg, AJ; Hampton, SL; Emsley, J; Stocks, MJ Discovery of α-Amidobenzylboronates as Highly Potent Covalent Inhibitors of Plasma Kallikrein. ACS Med Chem Lett 15: 501-509 (2024)
- Pelliccia, S; Cerchia, C; Esposito, F; Cannalire, R; Corona, A; Costanzi, E; Kuzikov, M; Gribbon, P; Zaliani, A; Brindisi, M; Storici, P; Tramontano, E; Summa, V Easy access to α-ketoamides as SARS-CoV-2 and MERS M Eur J Med Chem 244: (2022)
- Han Jeong, G; Cho, JH; Park, KI; Kim, K; Hoon Kim, T Enzymatic transformation of esculetin as a potent class of α-glucosidase inhibitors. Bioorg Med Chem Lett 88: (2023)
- Kim, SO; Han, Y; Ahn, S; An, S; Shin, JC; Choi, H; Kim, HJ; Park, NH; Kim, YJ; Jin, SH; Rho, HS; Noh, M Kojyl cinnamate esters are peroxisome proliferator-activated receptor α/γ dual agonists. Bioorg Med Chem 26: 5654-5663 (2018)
- Huang, JJ; Zhang, ZH; He, F; Liu, XW; Xu, XJ; Dai, LJ; Liu, QM; Yuan, M Novel naftopidil derivatives containing methyl phenylacetate and their blocking effects on α Bioorg Med Chem Lett 28: 547-551 (2018)
- Brear, P; North, A; Iegre, J; Hadje Georgiou, K; Lubin, A; Carro, L; Green, W; Sore, HF; Hyvönen, M; Spring, DR Novel non-ATP competitive small molecules targeting the CK2 α/β interface. Bioorg Med Chem 26: 3016-3020 (2018)
- Pacifico, S; Ferretti, V; Albanese, V; Fantinati, A; Gallerani, E; Nicoli, F; Gavioli, R; Zamberlan, F; Preti, D; Marastoni, M Synthesis and Biological Activity of Peptide α-Ketoamide Derivatives as Proteasome Inhibitors. ACS Med Chem Lett 10: 1086-1092 (2019)
- Schumann, NC; Bruning, J; Marshall, AC; Abell, AD The role of N-terminal heterocycles in hydrogen bonding to α-chymotrypsin. Bioorg Med Chem Lett 29: 396-399 (2019)
- Moreno-Cinos, C; Sassetti, E; Salado, IG; Witt, G; Benramdane, S; Reinhardt, L; Cruz, CD; Joossens, J; Van der Veken, P; Brötz-Oesterhelt, H; Tammela, P; Winterhalter, M; Gribbon, P; Windshügel, B; Augustyns, K α-Amino Diphenyl Phosphonates as Novel Inhibitors of Escherichia coli ClpP Protease. J Med Chem 62: 774-797 (2019)
- Palica, K; Deufel, F; Skagseth, S; Di Santo Metzler, GP; Thoma, J; Andersson Rasmussen, A; Valkonen, A; Sunnerhagen, P; Leiros, HS; Andersson, H; Erdelyi, M α-Aminophosphonate inhibitors of metallo-β-lactamases NDM-1 and VIM-2. RSC Med Chem 14: 2277-2300 (2023)
- Chu, W; Zhou, D; Gaba, V; Liu, J; Li, S; Peng, X; Xu, J; Dhavale, D; Bagchi, DP; d'Avignon, A; Shakerdge, NB; Bacskai, BJ; Tu, Z; Kotzbauer, PT; Mach, RH Design, Synthesis, and Characterization of 3-(Benzylidene)indolin-2-one Derivatives as Ligands fora-Synuclein Fibrils. J Med Chem 58: 6002-17 (2015)
- Wubshet, SG; Tahtah, Y; Heskes, AM; Kongstad, KT; Pateraki, I; Hamberger, B; Møller, BL; Staerk, D Identification of PTP1B and α-Glucosidase Inhibitory Serrulatanes from Eremophila spp. by Combined use of Dual High-Resolution PTP1B and α-Glucosidase Inhibition Profiling and HPLC-HRMS-SPE-NMR. J Nat Prod 79: 1063-72 (2016)
- Lumeras Amador, W; Boulet, SL; Burkholder, TP; Carballares Martin, S; Gilmour, R; Hahn, PJ; Bauer, RA; Rankovic, Z Mutant IDH1 inhibitors US Patent US10696665 (2020)
- Belgi, A; Burnley, JV; MacRaild, CA; Chhabra, S; Elnahriry, KA; Robinson, SD; Gooding, SG; Tae, HS; Bartels, P; Sadeghi, M; Zhao, FY; Wei, H; Spanswick, D; Adams, DJ; Norton, RS; Robinson, AJ Alkyne-Bridged α-Conotoxin Vc1.1 Potently Reverses Mechanical Allodynia in Neuropathic Pain Models. J Med Chem 64: 3222-3233 (2021)
- Wang, Z; Song, T; Feng, Y; Guo, Z; Fan, Y; Xu, W; Liu, L; Wang, A; Zhang, Z Bcl-2/MDM2 Dual Inhibitors Based on Universal Pyramid-Like α-Helical Mimetics. J Med Chem 59: 3152-62 (2016)
- Paudel, P; Pandey, P; Paris, JJ; Ashpole, NM; Mahdi, F; Tian, JM; Lee, J; Wang, M; Xu, M; Chittiboyina, AG; Khan, IA; Ross, SA; Li, XC Cannabinoid Receptor Type II Ligands from Sandalwood Oil and Synthetic α-Santalol Derivatives. J Nat Prod 86: 1786-1792 (2023)
- Zhang, XQ; Mou, XF; Mao, N; Hao, JJ; Liu, M; Zheng, JY; Wang, CY; Gu, YC; Shao, CL Design, semisynthesis, α-glucosidase inhibitory, cytotoxic, and antibacterial activities of p-terphenyl derivatives. Eur J Med Chem 146: 232-244 (2018)
- Barrett, SD; Holt, MC; Kramer, JB; Germain, B; Ho, CS; Ciske, FL; Kornilov, A; Colombo, JM; Uzieblo, A; O'Malley, JP; Owen, TA; Stein, AJ; Morano, MI Difluoromethylene at the γ-Lactam α-Position Improves 11-Deoxy-8-aza-PGE J Med Chem 62: 4731-4741 (2019)
- Huang, H; Meegalla, SK; Lanter, JC; Winters, MP; Zhao, S; Littrell, J; Qi, J; Rady, B; Lee, PS; Liu, J; Martin, T; Lam, WW; Xu, F; Lim, HK; Wilde, T; Silva, J; Otieno, M; Pocai, A; Player, MR Discovery of a GPR40 Superagonist: The Impact of Aryl Propionic Acid α-Fluorination. ACS Med Chem Lett 10: 16-21 (2019)
- Peng, L; Zhang, Z; Lei, C; Li, S; Zhang, Z; Ren, X; Chang, Y; Zhang, Y; Xu, Y; Ding, K Identification of New Small-Molecule Inducers of Estrogen-related Receptor α (ERRα) Degradation. ACS Med Chem Lett 10: 767-772 (2019)
- Mologni, L; Orsato, A; Zambon, A; Tardy, S; Bisson, WH; Schneider, C; Ceccon, M; Viltadi, M; D'Attoma, J; Pannilunghi, S; Vece, V; Gueyrard, D; Bertho, J; Scapozza, L; Goekjian, P; Gambacorti-Passerini, C Identification of non-ATP-competitive α-carboline inhibitors of the anaplastic lymphoma kinase. Eur J Med Chem 238: (2022)
- Coulup, SK; Huang, DS; Wong, HL; Georg, GI Identification of the Metabolic Profile of the α-Tubulin-Binding Natural Product (-)-Pironetin. J Med Chem 62: 1684-1689 (2019)
- Patch, RJ; Huang, H; Patel, S; Cheung, W; Xu, G; Zhao, BP; Beauchamp, DA; Rentzeperis, D; Geisler, JG; Askari, HB; Liu, J; Kasturi, J; Towers, M; Gaul, MD; Player, MR Indazole-based ligands for estrogen-related receptor α as potential anti-diabetic agents. Eur J Med Chem 138: 830-853 (2017)
- Sobati, M; Abdoli, M; Bonardi, A; Gratteri, P; Supuran, CT; Žalubovskis, R Inhibition Profiles of Some Novel Sulfonamide-Incorporated α-Aminophosphonates on Human Carbonic Anhydrases. ACS Med Chem Lett 14: 1067-1072 (2023)
- Kobzar, O; Shulha, Y; Buldenko, V; Cherenok, S; Silenko, O; Kalchenko, V; Vovk, A Inhibition of glutathione S-transferases by photoactive calix[4]arene α-ketophosphonic acids. Bioorg Med Chem Lett 77: (2022)
- Plut, E; Calderón, JC; Stanojlović, V; Gattor, AO; Höring, C; Humphrys, LJ; Konieczny, A; Kerres, S; Schubert, M; Keller, M; Cabrele, C; Clark, T; Reiser, O Stereochemistry-Driven Interactions of α,γ-Peptide Ligands with the Neuropeptide Y Y J Med Chem 66: 9642-9657 (2023)
- Scortichini, M; Idris, RM; Moschütz, S; Keim, A; Salmaso, V; Dobelmann, C; Oliva, P; Losenkova, K; Irjala, H; Vaittinen, S; Sandholm, J; Yegutkin, GG; Sträter, N; Junker, A; Müller, CE; Jacobson, KA Structure-Activity Relationship of 3-Methylcytidine-5'-α,β-methylenediphosphates as CD73 Inhibitors. J Med Chem 65: 2409-2433 (2022)
- Dong, J; Wang, Z; Cui, J; Meng, Q; Li, S Synthesis and structure-activity relationship studies of α-naphthoflavone derivatives as CYP1B1 inhibitors. Eur J Med Chem 187: (2020)
- Hoshikawa, T; Kurokawa, T; Yoshimura, H; Shibuguchi, T α-Fluorination of tropane compounds and its impact on physicochemical and ADME properties. Bioorg Med Chem Lett 108:
- Dos Santos, DA; Deobald, AM; Cornelio, VE; Ávila, RMD; Cornea, RC; Bernasconi, GCR; Paixão, MW; Vieira, PC; Corrêa, AG Asymmetric synthesis and evaluation of epoxy-α-acyloxycarboxamides as selective inhibitors of cathepsin L. Bioorg Med Chem 25: 4620-4627 (2017)
- Yu, R; Kompella, SN; Adams, DJ; Craik, DJ; Kaas, Q Determination of the α-conotoxin Vc1.1 binding site on the α9α10 nicotinic acetylcholine receptor. J Med Chem 56: 3557-67 (2013)
- Khan, P; Queen, A; Mohammad, T; Smita, na; Khan, NS; Hafeez, ZB; Hassan, MI; Ali, S Identification of α-Mangostin as a Potential Inhibitor of Microtubule Affinity Regulating Kinase 4. J Nat Prod 82: 2252-2261 (2019)
- Oulous, A; Daoudi, NE; Harit, T; Cherfi, M; Bnouham, M; Malek, F New pyrazole-tetrazole hybrid compounds as potent α-amylase and non-enzymatic glycation inhibitors. Bioorg Med Chem Lett 69: (2022)
- Liang, B; Xiao, D; Wang, SH; Xu, X Novel thiosemicarbazide-based β-carboline derivatives as α-glucosidase inhibitors: Synthesis and biological evaluation. Eur J Med Chem 275:
- Jung, KY; Wang, H; Teriete, P; Yap, JL; Chen, L; Lanning, ME; Hu, A; Lambert, LJ; Holien, T; Sundan, A; Cosford, ND; Prochownik, EV; Fletcher, S Perturbation of the c-Myc-Max protein-protein interaction via synthetic α-helix mimetics. J Med Chem 58: 3002-24 (2015)
- Son, S; Ko, SK; Jang, M; Lee, JK; Kwon, MC; Kang, DH; Ryoo, IJ; Lee, JS; Hong, YS; Kim, BY; Jang, JH; Ahn, JS Polyketides and Anthranilic Acid Possessing 6-Deoxy-α-l-talopyranose from a Streptomyces Species. J Nat Prod 80: 1378-1386 (2017)
- Qin, W; Xie, M; Qin, X; Fang, Q; Yin, F; Li, Z Recent advances in peptidomimetics antagonists targeting estrogen receptor α-coactivator interaction in cancer therapy. Bioorg Med Chem Lett 28: 2827-2836 (2018)
- Mushtaq, A; Azam, U; Mehreen, S; Naseer, MM Synthetic α-glucosidase inhibitors as promising anti-diabetic agents: Recent developments and future challenges. Eur J Med Chem 249: (2023)
- Sang, P; Shi, Y; Lu, J; Chen, L; Yang, L; Borcherds, W; Abdulkadir, S; Li, Q; Daughdrill, G; Chen, J; Cai, J α-Helix-Mimicking Sulfono-γ-AApeptide Inhibitors for p53-MDM2/MDMX Protein-Protein Interactions. J Med Chem 63: 975-986 (2020)
- Matthiesen, RA; Varney, ML; Xu, PC; Rier, AS; Wiemer, DF; Holstein, SA α-Methylation enhances the potency of isoprenoid triazole bisphosphonates as geranylgeranyl diphosphate synthase inhibitors. Bioorg Med Chem 26: 376-385 (2018)
- Kelly, PF; Collis, A; Davis, J; Walker, D; Ashwell, S; Thomson, B; Lu, W Inhibiting mutant IDH-1 US Patent US11376246 (2022)
- Li, A; Li, S; Wang, P; Dang, C; Liu, D KRAS mutant protein inhibitors US Patent US11345701 (2022)
- Wang, Y; Ye, R; Fan, L; Zhao, X; Li, L; Zheng, H; Qiu, Y; He, X; Lu, Y A TNF-α blocking peptide that reduces NF-κB and MAPK activity for attenuating inflammation. Bioorg Med Chem 92: (2023)
- Disch, JS; Duffy, JM; Lee, ECY; Gikunju, D; Chan, B; Levin, B; Monteiro, MI; Talcott, SA; Lau, AC; Zhou, F; Kozhushnyan, A; Westlund, NE; Mullins, PB; Yu, Y; von Rechenberg, M; Zhang, J; Arnautova, YA; Liu, Y; Zhang, Y; McRiner, AJ; Keefe, AD; Kohlmann, A; Clark, MA; Cuozzo, JW; Huguet, C; Arora, S Bispecific Estrogen Receptor α Degraders Incorporating Novel Binders Identified Using DNA-Encoded Chemical Library Screening. J Med Chem 64: 5049-5066 (2021)
- Bag, S; Ghosh, S; Tulsan, R; Sood, A; Zhou, W; Schifone, C; Foster, M; LeVine, H; Török, B; Török, M Design, synthesis and biological activity of multifunctional α,β-unsaturated carbonyl scaffolds for Alzheimer's disease. Bioorg Med Chem Lett 23: 2614-8 (2013)
- Luthra, T; Naga Lalitha, K; Agarwal, R; Uma, A; Sen, S Design, synthesis and in vitro study of densely functionalized oxindoles as potent α-glucosidase inhibitors. Bioorg Med Chem 26: 4996-5005 (2018)
- Hernández-Vázquez, E; Martínez-Caballero, S; Aldana-Torres, D; Estrada-Soto, S; Nieto-Camacho, A Discovery of dual-action phenolic 4-arylidene-isoquinolinones with antioxidant and α-glucosidase inhibition activities. RSC Med Chem 15: 519-538 (2024)
- Fairweather, AER; Goetz, DB; Schroeder, CM; Bhuiyan, NH; Varney, ML; Wiemer, DF; Holstein, SA Impact of α-modifications on the activity of triazole bisphosphonates as geranylgeranyl diphosphate synthase inhibitors. Bioorg Med Chem 44: (2021)
- Ann, J; Czikora, A; Saini, AS; Zhou, X; Mitchell, GA; Lewin, NE; Peach, ML; Blumberg, PM; Lee, J α-Arylidene Diacylglycerol-Lactones (DAG-Lactones) as Selective Ras Guanine-Releasing Protein 3 (RasGRP3) Ligands. J Med Chem 61: 6261-6276 (2018)
- Güzel-Akdemir, Ö; Akdemir, A; Pan, P; Vermelho, AB; Parkkila, S; Scozzafava, A; Capasso, C; Supuran, CT A class of sulfonamides with strong inhibitory action against the α-carbonic anhydrase from Trypanosoma cruzi. J Med Chem 56: 5773-81 (2013)
- Wang, J; Liang, B; Chen, Y; Fuk-Woo Chan, J; Yuan, S; Ye, H; Nie, L; Zhou, J; Wu, Y; Wu, M; Huang, LS; An, J; Warshel, A; Yuen, KY; Ciechanover, A; Huang, Z; Xu, Y A new class of α-ketoamide derivatives with potent anticancer and anti-SARS-CoV-2 activities. Eur J Med Chem 215: (2021)
- Shanmuganathan, B; Suryanarayanan, V; Sathya, S; Narenkumar, M; Singh, SK; Ruckmani, K; Pandima Devi, K Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur J Med Chem 143: 1196-1207 (2018)
- Li, QS; Li, CY; Lu, X; Zhang, H; Zhu, HL Design, synthesis and biological evaluation of novel (E)-α-benzylsulfonyl chalcone derivatives as potential BRAF inhibitors. Eur J Med Chem 50: 288-95 (2012)
- Singla, R; Gupta, KB; Upadhyay, S; Dhiman, M; Jaitak, V Design, synthesis and biological evaluation of novel indole-benzimidazole hybrids targeting estrogen receptor alpha (ER-α). Eur J Med Chem 146: 206-219 (2018)
- Abdu-Allah, HHM; Wu, SC; Lin, CH; Tseng, YY Design, synthesis and molecular docking study of α-triazolylsialosides as non-hydrolyzable and potent CD22 ligands. Eur J Med Chem 208: (2020)
- Guerlavais, V; Sawyer, TK; Carvajal, L; Chang, YS; Graves, B; Ren, JG; Sutton, D; Olson, KA; Packman, K; Darlak, K; Elkin, C; Feyfant, E; Kesavan, K; Gangurde, P; Vassilev, LT; Nash, HM; Vukovic, V; Aivado, M; Annis, DA Discovery of Sulanemadlin (ALRN-6924), the First Cell-Permeating, Stabilized α-Helical Peptide in Clinical Development. J Med Chem 66: 9401-9417 (2023)
- Dong, J; Huang, G; Cui, Q; Meng, Q; Li, S; Cui, J Discovery of heterocycle-containing α-naphthoflavone derivatives as water-soluble, highly potent and selective CYP1B1 inhibitors. Eur J Med Chem 209: (2021)
- Algar, S; Martín-Martínez, M; González-Muñiz, R Evolution in non-peptide α-helix mimetics on the road to effective protein-protein interaction modulators. Eur J Med Chem 211: (2021)
- Pieroni, M; Annunziato, G; Azzali, E; Dessanti, P; Mercurio, C; Meroni, G; Trifiró, P; Vianello, P; Villa, M; Beato, C; Varasi, M; Costantino, G Further insights into the SAR of α-substituted cyclopropylamine derivatives as inhibitors of histone demethylase KDM1A. Eur J Med Chem 92: 377-86 (2015)
- Xiao, D; Lu, L; Liang, B; Xiong, Z; Xu, X; Chen, WH Identification of 1,3,4-oxadiazolyl-containing β-carboline derivatives as novel α-glucosidase inhibitors with antidiabetic activity. Eur J Med Chem 261:
- Deligia, F; Murineddu, G; Gotti, C; Ragusa, G; Fasoli, F; Sciaccaluga, M; Plutino, S; Fucile, S; Loriga, G; Asproni, B; Pinna, GA Pyridinyl- and pyridazinyl-3,6-diazabicyclo[3.1.1]heptane-anilines: Novel selective ligands with subnanomolar affinity for α Eur J Med Chem 152: 401-416 (2018)
- Yu, J; Zhu, X; Harvey, PJ; Kaas, Q; Zhangsun, D; Craik, DJ; Luo, S Single Amino Acid Substitution in α-Conotoxin TxID Reveals a Specific α3β4 Nicotinic Acetylcholine Receptor Antagonist. J Med Chem 61: 9256-9265 (2018)
- Papaneophytou, CP; Mettou, AK; Rinotas, V; Douni, E; Kontopidis, GA Solvent Selection for Insoluble Ligands, a Challenge for Biological Assay Development: A TNF-α/SPD304 Study. ACS Med Chem Lett 4: 137-41 (2013)
- Wang, J; Su, S; Zhang, S; Zhai, S; Sheng, R; Wu, W; Guo, R Structure-activity relationship and synthetic methodologies of α-santonin derivatives with diverse bioactivities: A mini-review. Eur J Med Chem 175: 215-233 (2019)
- Oliva, P; Scortichini, M; Dobelmann, C; Jain, S; Gopinatth, V; Toti, KS; Phung, NB; Junker, A; Jacobson, KA Structure-activity relationships of pyrimidine nucleotides containing a 5'-α,β-methylene diphosphonate at the P2Y Bioorg Med Chem Lett 45: (2021)
- Handzlik, J; Szymańska, E; Wójcik, R; Dela, A; Jastrzębska-Więsek, M; Karolak-Wojciechowska, J; Fruziński, A; Siwek, A; Filipek, B; Kieć-Kononowicz, K Synthesis and SAR-study for novel arylpiperazine derivatives of 5-arylidenehydantoin with α₁-adrenoceptor antagonistic properties. Bioorg Med Chem 20: 4245-57 (2012)
- Zhang, W; Bai, H; Han, L; Zhang, H; Xu, B; Cui, J; Wang, X; Ge, Z; Li, R Synthesis and biological evaluation of curcumin derivatives modified with α-amino boronic acid as proteasome inhibitors. Bioorg Med Chem Lett 28: 2459-2464 (2018)
- Nocentini, A; Lucidi, A; Perut, F; Massa, A; Tomaselli, D; Gratteri, P; Baldini, N; Rotili, D; Mai, A; Supuran, CT α,γ-Diketocarboxylic Acids and Their Esters Act as Carbonic Anhydrase IX and XII Selective Inhibitors. ACS Med Chem Lett 10: 661-665 (2019)
- Sang, P; Zeng, H; Lee, C; Shi, Y; Wang, M; Pan, C; Wei, L; Huang, C; Wu, M; Shen, W; Li, X; Cai, J α/Sulfono-γ-AApeptide Hybrid Analogues of Glucagon with Enhanced Stability and Prolonged In Vivo Activity. J Med Chem 64: 13893-13901 (2021)
- Magnus, P; Ghavimi, B; Coe, JW Access to 7β-analogs of codeine with mixed μ/δ agonist activity via 6,7-α-epoxide opening. Bioorg Med Chem Lett 23: 4870-4 (2013)
- Cincinelli, A; Martellini, T; Vullo, D; Supuran, CT Anion and sulfonamide inhibition studies of an α-carbonic anhydrase from the Antarctic hemoglobinless fish Chionodraco hamatus. Bioorg Med Chem Lett 25: 5485-9 (2015)
- Mey, ASJS; Juárez-Jiménez, J; Hennessy, A; Michel, J Blinded predictions of binding modes and energies of HSP90-α ligands for the 2015 D3R grand challenge. Bioorg Med Chem 24: 4890-4899 (2016)
- Zheng, N; Christensen, SB; Blakely, A; Dowell, C; Purushottam, L; McIntosh, JM; Chou, DH Development of Conformationally Constrained α-RgIA Analogues as Stable Peptide Antagonists of Human α9α10 Nicotinic Acetylcholine Receptors. J Med Chem 63: 8380-8387 (2020)
- Liang, J; Huang, YY; Zhou, Q; Gao, Y; Li, Z; Wu, D; Yu, S; Guo, L; Chen, Z; Huang, L; Liang, SH; He, X; Wu, R; Luo, HB Discovery and Optimization of α-Mangostin Derivatives as Novel PDE4 Inhibitors for the Treatment of Vascular Dementia. J Med Chem 63: 3370-3380 (2020)
- Li, X; Huang, Y; Cheng, J; Zhang, L; Mao, F; Zhu, J; Sheng, C; Li, J Discovery of novel Syk/PDGFR-α/c-Kit inhibitors as multi-targeting drugs to treat rheumatoid arthritis. Bioorg Med Chem 26: 4375-4381 (2018)
- Liu, F; Cui, Y; Yang, F; Xu, Z; Da, LT; Zhang, Y Inhibition of polypeptide N-acetyl-α-galactosaminyltransferases is an underlying mechanism of dietary polyphenols preventing colorectal tumorigenesis. Bioorg Med Chem 27: 3372-3382 (2019)
- Plescia, J; De Cesco, S; Patrascu, MB; Kurian, J; Di Trani, J; Dufresne, C; Wahba, AS; Janmamode, N; Mittermaier, AK; Moitessier, N Integrated Synthetic, Biophysical, and Computational Investigations of Covalent Inhibitors of Prolyl Oligopeptidase and Fibroblast Activation Protein α. J Med Chem 62: 7874-7884 (2019)
- Kahraman, M; Govek, SP; Nagasawa, JY; Lai, A; Bonnefous, C; Douglas, K; Sensintaffar, J; Liu, N; Lee, K; Aparicio, A; Kaufman, J; Qian, J; Shao, G; Prudente, R; Joseph, JD; Darimont, B; Brigham, D; Heyman, R; Rix, PJ; Hager, JH; Smith, ND Maximizing ER-α Degradation Maximizes Activity in a Tamoxifen-Resistant Breast Cancer Model: Identification of GDC-0927. ACS Med Chem Lett 10: 50-55 (2019)
- Plescia, J; Hédou, D; Pousse, ME; Labarre, A; Dufresne, C; Mittermaier, A; Moitessier, N Modulating the selectivity of inhibitors for prolyl oligopeptidase inhibitors and fibroblast activation protein-α for different indications. Eur J Med Chem 240: (2022)
- Giampietro, L; Laghezza, A; Cerchia, C; Florio, R; Recinella, L; Capone, F; Ammazzalorso, A; Bruno, I; De Filippis, B; Fantacuzzi, M; Ferrante, C; Maccallini, C; Tortorella, P; Verginelli, F; Brunetti, L; Cama, A; Amoroso, R; Loiodice, F; Lavecchia, A Novel Phenyldiazenyl Fibrate Analogues as PPAR α/γ/δ Pan-Agonists for the Amelioration of Metabolic Syndrome. ACS Med Chem Lett 10: 545-551 (2019)
- Ortega, JA; Arencibia, JM; Minniti, E; Byl, JAW; Franco-Ulloa, S; Borgogno, M; Genna, V; Summa, M; Bertozzi, SM; Bertorelli, R; Armirotti, A; Minarini, A; Sissi, C; Osheroff, N; De Vivo, M Novel, Potent, and Druglike Tetrahydroquinazoline Inhibitor That Is Highly Selective for Human Topoisomerase II α over β. J Med Chem 63: 12873-12886 (2020)
- Ho, TNT; Lee, HS; Swaminathan, S; Goodwin, L; Rai, N; Ushay, B; Lewis, RJ; Rosengren, KJ; Conibear, AC Posttranslational modifications of α-conotoxins: sulfotyrosine and C-terminal amidation stabilise structures and increase acetylcholine receptor binding. RSC Med Chem 12: 1574-1584 (2021)
- Zhou, J; Mock, ED; Al Ayed, K; Di, X; Kantae, V; Burggraaff, L; Stevens, AF; Martella, A; Mohr, F; Jiang, M; van der Wel, T; Wendel, TJ; Ofman, TP; Tran, Y; de Koster, N; van Westen, GJP; Hankemeier, T; van der Stelt, M Structure-Activity Relationship Studies of α-Ketoamides as Inhibitors of the Phospholipase A and Acyltransferase Enzyme Family. J Med Chem 63: 9340-9359 (2020)
- Ullah, S; Kang, D; Lee, S; Ikram, M; Park, C; Park, Y; Yoon, S; Chun, P; Moon, HR Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in α-MSH-stimulated B16F10 melanoma cells. Eur J Med Chem 161: 78-92 (2019)
- Kamiyama, H; Kubo, Y; Sato, H; Yamamoto, N; Fukuda, T; Ishibashi, F; Iwao, M Synthesis, structure-activity relationships, and mechanism of action of anti-HIV-1 lamellarin α 20-sulfate analogues. Bioorg Med Chem 19: 7541-50 (2011)
- Chen, Z; Marcé, P; Resende, R; Alzari, PM; Frasch, AC; van den Elsen, JMH; Crennell, SJ; Watts, AG The synthesis and kinetic evaluation of aryl α-aminophosphonates as novel inhibitors of T. cruzi trans-sialidase. Eur J Med Chem 158: 25-33 (2018)
- Kim, SJ; Yang, J; Lee, S; Park, C; Kang, D; Akter, J; Ullah, S; Kim, YJ; Chun, P; Moon, HR The tyrosinase inhibitory effects of isoxazolone derivatives with a (Z)-β-phenyl-α, β-unsaturated carbonyl scaffold. Bioorg Med Chem 26: 3882-3889 (2018)
- ALVAREZ, FG; MANDAL, PK; HORTON, MC; SOTH, MJ; LE, K; JONES, P; MCAFOOS, TJ; SRIVASTAVA, P INHIBITORS OF MUTANT IDH1 AND IDH2 US Patent US20250049809 (2025)
- Lin, J; Luke, GP; Mondal, M Inhibiting mutant isocitrate dehydrogenase 1 (mIDH1) US Patent US11566013 (2023)
- Dinda, B; Das, B; Biswas, S; Sharma, H; Armstrong, C; Yedlapudi, D; Antonio, T; Reith, M; Dutta, AK Bivalent dopamine agonists with co-operative binding and functional activities at dopamine D2 receptors, modulate aggregation and toxicity of alpha synuclein protein. Bioorg Med Chem 78: (2023)
- Bhattarai, S; Pippel, J; Scaletti, E; Idris, R; Freundlieb, M; Rolshoven, G; Renn, C; Lee, SY; Abdelrahman, A; Zimmermann, H; El-Tayeb, A; Müller, CE; Sträter, N 2-Substituted α,β-Methylene-ADP Derivatives: Potent Competitive Ecto-5'-nucleotidase (CD73) Inhibitors with Variable Binding Modes. J Med Chem 63: 2941-2957 (2020)
- Zhang, L; Lin, D; Sun, X; Curth, U; Drosten, C; Sauerhering, L; Becker, S; Rox, K; Hilgenfeld, R Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368: 409-412 (2020)
- Chahal, S; Punia, J; Rani, P; Singh, R; Mayank, na; Kumar, P; Kataria, R; Joshi, G; Sindhu, J Development of thiazole-appended novel hydrazones as a new class of α-amylase inhibitors with anticancer assets: an RSC Med Chem 14: 757-781 (2023)
- Huang, C; Zeng, R; Qiao, J; Quan, B; Luo, R; Huang, Q; Guo, N; Li, Y; Long, X; Ma, R; Xia, A; Fang, Z; Wang, Y; Li, Y; Zheng, Y; Li, L; Lei, J; Yang, S Discovery and structure-activity relationship studies of novel α-ketoamide derivatives targeting the SARS-CoV-2 main protease. Eur J Med Chem 259:
- Xu, T; Meng, JR; Cheng, W; Liu, JZ; Chu, J; Zhang, Q; Ma, N; Bai, LP; Guo, Y Discovery of honokiol thioethers containing 1,3,4-oxadiazole moieties as potential α-glucosidase and SARS-CoV-2 entry inhibitors. Bioorg Med Chem 67: (2022)
- Chen, X; Li, P; Huang, J; Yang, Y; Zhang, H; Wang, Z; Zhu, Z; Wang, J; Zhang, J; Chen, K; He, H; Long, C; Chen, S Discovery of novel bicyclic[3.3.0]proline peptidyl α-ketoamides as potent 3CL-protease inhibitors for SARS-CoV-2. Bioorg Med Chem Lett 90: (2023)
- Hanisak, J; Soriano, A; Adam, GC; Basso, A; Bauman, D; Bell, D; Frank, E; O'Donnell, G; Tawa, P; Verras, A; Yu, Y; Zhang, L; Seganish, WM Discovery of the First Non-cGMP Mimetic Small Molecule Activators of cGMP-Dependent Protein Kinase 1 α (PKG1α). ACS Med Chem Lett 12: 1275-1282 (2021)
- Martucci, WE; Rodriguez, JM; Vargo, MA; Marr, M; Hamilton, AD; Anderson, KS Exploring novel strategies for AIDS protozoal pathogens: α-helix mimetics targeting a key allosteric protein-protein interaction in Medchemcomm 4: (2013)
- Liang, J; Tae, HS; Zhao, Z; Li, X; Zhang, J; Chen, S; Jiang, T; Adams, DJ; Yu, R Mechanism of Action and Structure-Activity Relationship of α-Conotoxin Mr1.1 at the Human α9α10 Nicotinic Acetylcholine Receptor. J Med Chem 65: 16204-16217 (2022)
- Yu, R; Tae, HS; Tabassum, N; Shi, J; Jiang, T; Adams, DJ Molecular Determinants Conferring the Stoichiometric-Dependent Activity of α-Conotoxins at the Human α9α10 Nicotinic Acetylcholine Receptor Subtype. J Med Chem 61: 4628-4634 (2018)
- Apaza Ticona, L; Sánchez Sánchez-Corral, J; Flores Sepúlveda, A; Soriano Vázquez, C; Hernán Vieco, C; Rumbero Sánchez, Á Novel 1,2,4-oxadiazole compounds as PPAR-α ligand agonists: a new strategy for the design of antitumour compounds. RSC Med Chem 14: 1377-1388 (2023)
- Zhao, C; Tang, C; Li, C; Ning, W; Hu, Z; Xin, L; Zhou, HB; Huang, J Novel hybrid conjugates with dual estrogen receptor α degradation and histone deacetylase inhibitory activities for breast cancer therapy. Bioorg Med Chem 40: (2021)
- Toh, ZS; Wang, H; Yip, YM; Lu, Y; Lim, BJ; Zhang, D; Huang, D Phenolic group on A-ring is key for dracoflavan B as a selective noncompetitive inhibitor of α-amylase. Bioorg Med Chem 23: 7641-9 (2015)
- Chu, J; Yang, R; Cheng, W; Cui, L; Pan, H; Liu, J; Guo, Y Semisynthesis, biological activities, and mechanism studies of Mannich base analogues of magnolol/honokiol as potential α-glucosidase inhibitors. Bioorg Med Chem 75: (2022)
- Okazaki, S; Shioi, R; Noguchi-Yachide, T; Ishikawa, M; Makishima, M; Hashimoto, Y; Yamaguchi, T Structure-activity relationship studies of non-carboxylic acid peroxisome proliferator-activated receptor α/δ (PPARα/δ) dual agonists. Bioorg Med Chem 24: 5455-5461 (2016)
- He, M; Li, YJ; Shao, J; Li, YS; Cui, ZN Synthesis and biological evaluation of 2,5-disubstituted furan derivatives containing 1,3-thiazole moiety as potential α-glucosidase inhibitors. Bioorg Med Chem Lett 83: (2023)
- Hu, C; Liang, B; Sun, J; Li, J; Xiong, Z; Wang, SH; Xuetao, X Synthesis and biological evaluation of indole derivatives containing thiazolidine-2,4-dione as α-glucosidase inhibitors with antidiabetic activity. Eur J Med Chem 264:
- Nguyen, HT; Tuan, AN; Thi, TAD; Van, KT; Le-Nhat-Thuy, G; Thi, PH; Thi, QGN; Thi, CB; Quang, HT; Van Nguyen, T Synthesis, in vitro Α-Glucosidase, and acetylcholinesterase inhibitory activities of novel Indol-Fused Pyrano[2,3-D]Pyrimidine compounds. Bioorg Med Chem Lett 98: (2024)
- Ruiz-Santaquiteria, M; de Castro, S; Toro, MA; de Lucio, H; Gutiérrez, KJ; Sánchez-Murcia, PA; Jiménez, MÁ; Gago, F; Jiménez-Ruiz, A; Camarasa, MJ; Velázquez, S Trypanothione reductase inhibition and anti-leishmanial activity of all-hydrocarbon stapled α-helical peptides with improved proteolytic stability. Eur J Med Chem 149: 238-247 (2018)
- Sugaya, K; Ino, M; Matsuo, N; Onose, JI; Abe, N Variegatic acid from the edible mushroom Tylopilus ballouii inhibits TNF-α production and PKCβ1 activity in leukemia cells. Bioorg Med Chem Lett 30: (2020)
- Gavale, KS; Chavan, SR; Kumbhar, N; Kawade, S; Doshi, P; Khan, A; Dhavale, DD α-Geminal disubstituted pyrrolidine iminosugars and their C-4-fluoro analogues: Synthesis, glycosidase inhibition and molecular docking studies. Bioorg Med Chem 25: 5148-5159 (2017)
- PubChem, PC Clearance of Mutant Huntingtin Protein - Confirmatory screen PubChem Bioassay (2007)
- Kelly, PF; Ashwell, S; Thomson, B; Collis, A; Davis, J; Walker, D; Lu, W Inhibiting mutant isocitrate dehydrogenase 1 (mIDH-1) US Patent US11311527 (2022)
- Sugiyama, A; Doi, H; Kodama, T; Inoue, T; Mizohata, E; Kawato, T; Meshizuka, T; Kanai, M; Shimizu, Y; Yamamoto, T Modified biotin, mutant streptavidin, and use thereof US Patent US9670255 (2017)
- Lee, K; Niu, D; Baevsky, MF Mutant-selective EGFR inhibitors and uses thereof US Patent US9409887 (2016)
- Sloman, DL; Chessari, G; Schöpf, P; Howard, S; Kawai, Y; Shibata, K; Asakura, H; Uno, T; Sagara, T; Nakamura, M; Kobayakawa, Y; Bennett, DJ; Bharathan, I; Graham, TH; Han, Y; Hussain, Z; Ma, X; Mandal, M; Otte, RD; Palani, A; Swaminathan, U; Uehling, M; Ye, Y; Chau, R; Christian, AH; Gathiaka, S; Henderson, TJ; Hennessy, ET; Hoover, AJ; Kawamura, S; Kolaj, I; Lyons, TW; Mitcheltree, MJ; Sather, A SMALL MOLECULE INHIBITORS OF KRAS G12D MUTANT US Patent US20240239788 (2024)
- Bharathan, I; Gathiaka, S; Han, Y; Ma, X; Otte, RD; Sloman, DL; Graham, TH; Henderson, T; Hennessy, E; Palani, A Small Molecule Inhibitors of KRAS G12C Mutant US Patent US20240043448 (2024)
- Kargbo, RB Small Molecule Inhibitors of KRAS G12C Mutant. ACS Med Chem Lett 12: 1210-1211 (2021)
- Feng, Y; Park, J; Li, SG; Boutin, R; Viereck, P; Schilling, MA; Berghuis, AM; Tsantrizos, YS Chirality-Driven Mode of Binding of α-Aminophosphonic Acid-Based Allosteric Inhibitors of the Human Farnesyl Pyrophosphate Synthase (hFPPS). J Med Chem 62: 9691-9702 (2019)
- Lu, L; Chen, J; Tao, W; Wang, Z; Liu, D; Zhou, J; Wu, X; Sun, H; Li, W; Tanabe, G; Muraoka, O; Zhao, B; Wu, L; Xie, W Design and Synthesis of Sulfonium Derivatives: A Novel Class of α-Glucosidase Inhibitors with Potent In Vivo Antihyperglycemic Activities. J Med Chem 66: 3484-3498 (2023)
- Ye, GJ; Lan, T; Huang, ZX; Cheng, XN; Cai, CY; Ding, SM; Xie, ML; Wang, B Design and synthesis of novel xanthone-triazole derivatives as potential antidiabetic agents: α-Glucosidase inhibition and glucose uptake promotion. Eur J Med Chem 177: 362-373 (2019)
- Axford, LC; Agarwal, PK; Anderson, KH; Andrau, LN; Atherall, J; Barker, S; Bennett, JM; Blair, M; Collins, I; Czaplewski, LG; Davies, DT; Gannon, CT; Kumar, D; Lancett, P; Logan, A; Lunniss, CJ; Mitchell, DR; Offermann, DA; Palmer, JT; Palmer, N; Pitt, GR; Pommier, S; Price, D; Narasinga Rao, B; Saxena, R; Shukla, T; Singh, AK; Singh, M; Srivastava, A; Steele, C; Stokes, NR; Thomaides-Brears, HB; Tyndall, EM; Watson, D; Haydon, DJ Design, synthesis and biological evaluation of α-substituted isonipecotic acid benzothiazole analogues as potent bacterial type II topoisomerase inhibitors. Bioorg Med Chem Lett 23: 6598-603 (2013)
- Liang, X; Zhang, X; Lu, X; Zheng, Z; Ma, X; Qi, S Diketopiperazine-Type Alkaloids from a Deep-Sea-Derived Aspergillus puniceus Fungus and Their Effects on Liver X Receptor α. J Nat Prod 82: 1558-1564 (2019)
- Rescourio, G; Gonzalez, AZ; Jabri, S; Belmontes, B; Moody, G; Whittington, D; Huang, X; Caenepeel, S; Cardozo, M; Cheng, AC; Chow, D; Dou, H; Jones, A; Kelly, RC; Li, Y; Lizarzaburu, M; Lo, MC; Mallari, R; Meleza, C; Rew, Y; Simonovich, S; Sun, D; Turcotte, S; Yan, X; Wong, SG; Yanez, E; Zancanella, M; Houze, J; Medina, JC; Hughes, PE; Brown, SP Discovery and in Vivo Evaluation of Macrocyclic Mcl-1 Inhibitors Featuring an α-Hydroxy Phenylacetic Acid Pharmacophore or Bioisostere. J Med Chem 62: 10258-10271 (2019)
- Vourloumis, D; Mavridis, I; Athanasoulis, A; Temponeras, I; Koumantou, D; Giastas, P; Mpakali, A; Magrioti, V; Leib, J; van Endert, P; Stratikos, E; Papakyriakou, A Discovery of Selective Nanomolar Inhibitors for Insulin-Regulated Aminopeptidase Based on α-Hydroxy-β-amino Acid Derivatives of Bestatin. J Med Chem 65: 10098-10117 (2022)
- Yu, DD; Van Citters, G; Li, H; Stoltz, BM; Forman, BM Discovery of novel modulators for the PPARα (peroxisome proliferator activated receptor α): Potential therapies for nonalcoholic fatty liver disease. Bioorg Med Chem 41: (2021)
- Sakauchi, N; Furukawa, H; Shirai, J; Sato, A; Kuno, H; Saikawa, R; Yoshida, M Identification of 3,4-dihydro-2H-thiochromene 1,1-dioxide derivatives with a phenoxyethylamine group as highly potent and selective α Eur J Med Chem 139: 114-127 (2017)
- Li, ZP; Song, YH; Uddin, Z; Wang, Y; Park, KH Inhibition of protein tyrosine phosphatase 1B (PTP1B) and α-glucosidase by xanthones from Cratoxylum cochinchinense, and their kinetic characterization. Bioorg Med Chem 26: 737-746 (2018)
- Zi, D; Song, YY; Lu, TT; Kise, M; Kato, A; Wang, JZ; Jia, YM; Li, YX; Fleet, GWJ; Yu, CY Nanomolar β-glucosidase and β-galactosidase inhibition by enantiomeric α-1-C-alkyl-1,4-dideoxy-1,4-imino-arabinitol derivatives. Eur J Med Chem 247: (2023)
- Moghadam Farid, S; Iraji, A; Mojtabavi, S; Ghasemi, M; Faramarzi, MA; Mahdavi, M; Barazandeh Tehrani, M; Akbarzadeh, T; Saeedi, M Quinazolinone-1,2,3-triazole-acetamide conjugates as potent α-glucosidase inhibitors: synthesis, enzyme inhibition, kinetic analysis, and molecular docking study. RSC Med Chem 14: 520-533 (2023)
- Šimková, A; Ormsby, T; Sidej, N; Slavětínská, LP; Brynda, J; Beranová, J; Šácha, P; Majer, P; Konvalinka, J Structure-activity relationship and biochemical evaluation of novel fibroblast activation protein and prolyl endopeptidase inhibitors with α-ketoamide warheads. Eur J Med Chem 224: (2021)
- Luo, HJ; Si, DJ; Sun, XJ; Wang, MY; Yang, YB; Wang, B; Wen, HM; Li, W; Liu, J Structure-based discovery of novel α-aminoketone derivatives as dual p53-MDM2/MDMX inhibitors for the treatment of cancer. Eur J Med Chem 252: (2023)
- Yelamanda Rao, K; Jeelan Basha, S; Monika, K; Sreelakshmi, M; Sivakumar, I; Mallikarjuna, G; Yadav, RM; Kumar, S; Subramanyam, R; Damu, AG Synthesis and anti-Alzheimer potential of novel α-amino phosphonate derivatives and probing their molecular interaction mechanism with acetylcholinesterase. Eur J Med Chem 253: (2023)
- Nielsen, AJ; Raez-Villanueva, S; Crankshaw, DJ; Holloway, AC; McNulty, J Synthesis of α-methylstilbenes using an aqueous Wittig methodology and application toward the development of potent human aromatase inhibitors. Bioorg Med Chem Lett 29: 1395-1398 (2019)
- Chung, S; Himmel, DM; Jiang, JK; Wojtak, K; Bauman, JD; Rausch, JW; Wilson, JA; Beutler, JA; Thomas, CJ; Arnold, E; Le Grice, SF Synthesis, activity, and structural analysis of novel α-hydroxytropolone inhibitors of human immunodeficiency virus reverse transcriptase-associated ribonuclease H. J Med Chem 54: 4462-73 (2011)
- Anananuchatkul, T; Tsutsumi, H; Miki, T; Mihara, H hDM2 protein-binding peptides screened from stapled α-helical peptide phage display libraries with different types of staple linkers. Bioorg Med Chem Lett 30: (2020)
- Pellicciari, R; Liscio, P; Giacchè, N; De Franco, F; Carotti, A; Robertson, J; Cialabrini, L; Katsyuba, E; Raffaelli, N; Auwerx, J α-Amino-β-carboxymuconate-ε-semialdehyde Decarboxylase (ACMSD) Inhibitors as Novel Modulators of De Novo Nicotinamide Adenine Dinucleotide (NAD J Med Chem 61: 745-759 (2018)
- Popovici-Muller, J; Lemieux, RM; Artin, E; Saunders, JO; Salituro, FG; Travins, J; Cianchetta, G; Cai, Z; Zhou, D; Cui, D; Chen, P; Straley, K; Tobin, E; Wang, F; David, MD; Penard-Lacronique, V; Quivoron, C; Saada, V; de Botton, S; Gross, S; Dang, L; Yang, H; Utley, L; Chen, Y; Kim, H; Jin, S; Gu, Z; Yao, G; Luo, Z; Lv, X; Fang, C; Yan, L; Olaharski, A; Silverman, L; Biller, S; Su, SM; Yen, K Discovery of AG-120 (Ivosidenib): A First-in-Class Mutant IDH1 Inhibitor for the Treatment of IDH1 Mutant Cancers. ACS Med Chem Lett 9: 300-305 (2018)
- Pan, P; Vermelho, AB; Scozzafava, A; Parkkila, S; Capasso, C; Supuran, CT Anion inhibition studies of the α-carbonic anhydrase from the protozoan pathogen Trypanosoma cruzi, the causative agent of Chagas disease. Bioorg Med Chem 21: 4472-6 (2013)
- De Marco, R; Bedini, A; Spampinato, S; Comellini, L; Zhao, J; Artali, R; Gentilucci, L Constraining Endomorphin-1 by β,α-Hybrid Dipeptide/Heterocycle Scaffolds: Identification of a Novel κ-Opioid Receptor Selective Partial Agonist. J Med Chem 61: 5751-5757 (2018)
- Liang, J; Tae, HS; Xu, X; Jiang, T; Adams, DJ; Yu, R Dimerization of α-Conotoxins as a Strategy to Enhance the Inhibition of the Human α7 and α9α10 Nicotinic Acetylcholine Receptors. J Med Chem 63: 2974-2985 (2020)
- Hou, Y; Zhang, F; Min, W; Yuan, K; Kuang, W; Wang, X; Zhu, Y; Sun, C; Xia, F; Wang, Y; Zhang, H; Wang, L; Yang, P Discovery of Novel Phosphoinositide-3-Kinase α Inhibitors with High Selectivity, Excellent Bioavailability, and Long-Acting Efficacy for Gastric Cancer. J Med Chem 65: 9873-9892 (2022)
- Jiang, Z; Liu, X; Yuan, Z; He, H; Wang, J; Zhang, X; Gong, Z; Hou, L; Shen, L; Guo, F; Zhang, J; Wang, J; Xu, D; Liu, Z; Li, H; Chen, X; Long, C; Li, J; Chen, S Discovery of a Novel Selective Dual Peroxisome Proliferator-Activated Receptor α/δ Agonist for the Treatment of Primary Biliary Cirrhosis. ACS Med Chem Lett 10: 1068-1073 (2019)
- Zhu, X; Pan, S; Xu, M; Zhang, L; Yu, J; Yu, J; Wu, Y; Fan, Y; Li, H; Kasheverov, IE; Kudryavtsev, DS; Tsetlin, VI; Xue, Y; Zhangsun, D; Wang, X; Luo, S High Selectivity of an α-Conotoxin LvIA Analogue for α3β2 Nicotinic Acetylcholine Receptors Is Mediated by β2 Functionally Important Residues. J Med Chem 63: 13656-13668 (2020)
- Kobayashi, JI; Hirasawa, H; Fujimori, Y; Nakanishi, O; Kamada, N; Ikeda, T; Yamamoto, A; Kanbe, H Identification of N-acyl-N-indanyl-α-phenylglycinamides as selective TRPM8 antagonists designed to mitigate the risk of adverse effects. Bioorg Med Chem 30: (2021)
- Golani, LK; Yeunus Mian, M; Ahmed, T; Pandey, KP; Mondal, P; Sharmin, D; Rezvanian, S; Witkin, JM; Cook, JM Rationalizing the binding and α subtype selectivity of synthesized imidazodiazepines and benzodiazepines at GABAA receptors by using molecular docking studies. Bioorg Med Chem Lett 62: (2022)
- Feng, Q; Zhang, J; Luo, S; Huang, Y; Peng, Z; Wang, G Synthesis, biological evaluation and action mechanism of 7H-[1,2,4] triazolo [3,4-b] [1,3,4] thiadiazine-phenylhydrazone derivatives as α-glucosidase inhibitors. Eur J Med Chem 262:
- Saito, S; Itoh, M; Fujisawa, T; Saito, H; Kiyotsuka, Y; Watanabe, H; Matsunaga, H; Kagoshima, Y; Suzuki, T; Ogawara, Y; Kitabayashi, K Isoxazole derivative as mutant isocitrate dehydrogenase 1 inhibitor US Patent US10040791 (2018)
- Lin, J; Ericsson, A; Campbell, A; Gustafson, G; Wang, Z; Diebold, RB; Ashwell, S; Lancia, Jr., DR; Caravella, JA; Lu, W Pyridinyl quinolinone derivatives as mutant-isocitrate dehydrogenase inhibitors US Patent US10253015 (2019)
- Lin, J; Ericsson, A; Campbell, A; Gustafson, G; Wang, Z; Diebold, RB; Ashwell, S; Lancia, Jr., DR; Caravella, JA; Lu, W Quinolinone pyrimidines compositions as mutant-isocitrate dehydrogenase inhibitors US Patent US10280150 (2019)
- Fischer, C; Bogen, SL; Childers, ML; Fradera Llinas, FX; Ellis, JM; Esposite, S; Hoffman, DM; Huang, C; Kattar, SD; Kim, AJ; Lampe, JW; Machacek, MR; McMasters, DR; Parker, Jr., DL; Reutershan, MH; Sciammetta, N; Shao, PP; Sloman, DL; Sun, W; Ujjainwalla, F; Wu, Z; Yu, Y; Gibeau, CR Tricyclic compounds as inhibitors of mutant IDH enzymes US Patent US10508108 (2019)
- Caciolla, J; Martini, S; Spinello, A; Pavlin, M; Turrini, E; Simonelli, F; Belluti, F; Rampa, A; Bisi, A; Fimognari, C; Zaffaroni, N; Gobbi, S; Magistrato, A Balanced dual acting compounds targeting aromatase and estrogen receptor α as an emerging therapeutic opportunity to counteract estrogen responsive breast cancer. Eur J Med Chem 224: (2021)
- Wang, C; Zhao, L; Xia, S; Zhang, T; Cao, R; Liang, G; Li, Y; Meng, G; Wang, W; Shi, W; Zhong, W; Jiang, S; Liu, K De Novo Design of α-Helical Lipopeptides Targeting Viral Fusion Proteins: A Promising Strategy for Relatively Broad-Spectrum Antiviral Drug Discovery. J Med Chem 61: 8734-8745 (2018)
- Ogasawara, D; Ichu, TA; Jing, H; Hulce, JJ; Reed, A; Ulanovskaya, OA; Cravatt, BF Discovery and Optimization of Selective and in Vivo Active Inhibitors of the Lysophosphatidylserine Lipase α/β-Hydrolase Domain-Containing 12 (ABHD12). J Med Chem 62: 1643-1656 (2019)
- Xie, B; Yin, Z; Hu, Z; Lv, J; Du, C; Deng, X; Huang, Y; Li, Q; Huang, J; Liang, K; Zhou, HB; Dong, C Discovery of a Novel Class of PROTACs as Potent and Selective Estrogen Receptor α Degraders to Overcome Endocrine-Resistant Breast Cancer J Med Chem 66: 6631-6651 (2023)
- Singh, A; Singh, K; Kaur, K; Sharma, A; Mohana, P; Prajapati, J; Kaur, U; Goswami, D; Arora, S; Chadha, R; Bedi, PMS Discovery of triazole tethered thymol/carvacrol-coumarin hybrids as new class of α-glucosidase inhibitors with potent in vivo antihyperglycemic activities. Eur J Med Chem 263:
- Deng, X; Deng, X; Ning, W; Xin, L; Li, Q; Hu, Z; Xie, B; Liang, K; Min, C; Dong, C; Huang, J; Zhou, HB Identification of Novel Dual-Target Estrogen Receptor α Degraders with Tubulin Inhibitory Activity for the Treatment of Endocrine-Resistant Breast Cancer. J Med Chem 66: 11094-11117 (2023)
- de Lucio, H; Gamo, AM; Ruiz-Santaquiteria, M; de Castro, S; Sánchez-Murcia, PA; Toro, MA; Gutiérrez, KJ; Gago, F; Jiménez-Ruiz, A; Camarasa, MJ; Velázquez, S Improved proteolytic stability and potent activity against Leishmania infantum trypanothione reductase of α/β-peptide foldamers conjugated to cell-penetrating peptides. Eur J Med Chem 140: 615-623 (2017)
- Bai, B; Belovodskiy, A; Hena, M; Kandadai, AS; Joyce, MA; Saffran, HA; Shields, JA; Khan, MB; Arutyunova, E; Lu, J; Bajwa, SK; Hockman, D; Fischer, C; Lamer, T; Vuong, W; van Belkum, MJ; Gu, Z; Lin, F; Du, Y; Xu, J; Rahim, M; Young, HS; Vederas, JC; Tyrrell, DL; Lemieux, MJ; Nieman, JA Peptidomimetic α-Acyloxymethylketone Warheads with Six-Membered Lactam P1 Glutamine Mimic: SARS-CoV-2 3CL Protease Inhibition, Coronavirus Antiviral Activity, and J Med Chem (2021)
- Yoshioka, Y; Namiki, D; Makiuchi, M; Sugaya, K; Onose, J; Ashida, H; Abe, N Vialinin A and thelephantin G, potent inhibitors of tumor necrosis factor-α production, inhibit sentrin/SUMO-specific protease 1 enzymatic activity. Bioorg Med Chem Lett 26: 4237-40 (2016)
- Tomita, T; Wadhwa, R; Kaul, SC; Kurita, R; Kojima, N; Onishi, Y Withanolide Derivative 2,3-Dihydro-3β-methoxy Withaferin-A Modulates the Circadian Clock via Interaction with RAR-Related Orphan Receptor α (RORa). J Nat Prod 84: 1882-1888 (2021)
- Dominguez, C; Bard, J; Lee, MR; Liu, L; Prime, ME; Coe, S Compounds for targeting mutant huntingtin protein and uses thereof US Patent US11389438 (2022)
- Zhou, T; Parillon, L; Li, F; Wang, Y; Keats, J; Lamore, S; Xu, Q; Shakespeare, W; Dalgarno, D; Zhu, X Crystal structure of the T315I mutant of AbI kinase. Chem Biol Drug Des 70: 171-81 (2007)
- Zheng, B; Yao, Y; Liu, Z; Deng, L; Anglin, JL; Jiang, H; Prasad, BV; Song, Y Crystallographic Investigation and Selective Inhibition of Mutant Isocitrate Dehydrogenase. ACS Med Chem Lett 4: 542-546 (2013)
- Abdel-Magid, AF Inhibitors of Mutant IDH for the Treatment of Cancer. ACS Med Chem Lett 5: 1184-5 (2014)
- Kaoud, TS; Mohassab, AM; Hassan, HA; Yan, C; Van Ravenstein, SX; Abdelhamid, D; Dalby, KN; Abdel-Aziz, M NO-releasing STAT3 inhibitors suppress BRAF-mutant melanoma growth. Eur J Med Chem 186: (2020)
- Zhou, W; Ercan, D; Chen, L; Yun, CH; Li, D; Capelletti, M; Cortot, AB; Chirieac, L; Iacob, RE; Padera, R; Engen, JR; Wong, KK; Eck, MJ; Gray, NS; Jänne, PA Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature 462: 1070-4
- BOMIO-CONFAGLIA, C; BRACHMANN, SM; COTESTA, S; GERSPACHER, M; LEBLANC, C; LIMA, F; LORTHIOIS, EL; MACHAUER, R; MAH, R; RACINE, S; RIGOLLIER, P; STUTZ, S; VAUPEL, A; WARIN, N; WILCKEN, R; ZECRI, F PYRAZOLYL DERIVATIVES AS INHIBITORS OF THE KRAS MUTANT PROTEIN US Patent US20240317721 (2024)
- Zhou, L; Chen, W; Cao, C; Shi, Y; Ye, W; Hu, J; Wang, L; Zhou, W Design and synthesis of α-naphthoflavone chimera derivatives able to eliminate cytochrome P450 (CYP)1B1-mediated drug resistance via targeted CYP1B1 degradation. Eur J Med Chem 189: (2020)
- Yang, J; Shibu, MA; Kong, L; Luo, J; BadrealamKhan, F; Huang, Y; Tu, ZC; Yun, CH; Huang, CY; Ding, K; Lu, X Design, Synthesis, and Structure-Activity Relationships of 1,2,3-Triazole Benzenesulfonamides as New Selective Leucine-Zipper and Sterile-α Motif Kinase (ZAK) Inhibitors. J Med Chem 63: 2114-2130 (2020)
- Wang, H; Wang, J; Zhao, X; Ye, R; Sun, L; Wang, J; Li, L; Liang, H; Wang, S; Lu, Y Discovery of an Anti-TNF-α 9-mer Peptide from a T7 Phage Display Library for the Treatment of Inflammatory Bowel Disease. J Med Chem 66: 6981-6993 (2023)
- Legru, A; Batista, FA; Puszko, AK; Bouillon, A; Maurel, M; Martinez, M; Ejjoummany, A; Ortega Varga, L; Adler, P; Méchaly, A; Hadjadj, M; Sosnowski, P; Hopfgartner, G; Alzari, PM; Blondel, A; Haouz, A; Barale, JC; Hernandez, JF Insights from structure-activity relationships and the binding mode of peptidic α-ketoamide inhibitors of the malaria drug target subtilisin-like SUB1. Eur J Med Chem 269:
- Carrieri, A; Giudici, M; Parente, M; De Rosas, M; Piemontese, L; Fracchiolla, G; Laghezza, A; Tortorella, P; Carbonara, G; Lavecchia, A; Gilardi, F; Crestani, M; Loiodice, F Molecular determinants for nuclear receptors selectivity: chemometric analysis, dockings and site-directed mutagenesis of dual peroxisome proliferator-activated receptors α/γ agonists. Eur J Med Chem 63: 321-32 (2013)
- McCullough, C; Neumann, TS; Gone, JR; He, Z; Herrild, C; Wondergem Nee Lukesh, J; Pandey, RK; Donaldson, WA; Sem, DS Probing the human estrogen receptor-α binding requirements for phenolic mono- and di-hydroxyl compounds: a combined synthesis, binding and docking study. Bioorg Med Chem 22: 303-10 (2014)
- Pickett, JE; Nagakura, K; Pasternak, AR; Grinnell, SG; Majumdar, S; Lewis, JS; Pasternak, GW Sandmeyer reaction repurposed for the site-selective, non-oxidizing radioiodination of fully-deprotected peptides: studies on the endogenous opioid peptide α-neoendorphin. Bioorg Med Chem Lett 23: 4347-50 (2013)
- Hendy, MS; Ali, AA; Ahmed, L; Hossam, R; Mostafa, A; Elmazar, MM; Naguib, BH; Attia, YM; Ahmed, MS Structure-based drug design, synthesis, In vitro, and In vivo biological evaluation of indole-based biomimetic analogs targeting estrogen receptor-α inhibition. Eur J Med Chem 166: 281-290 (2019)
- Pandit, SS; Kulkarni, MR; Pandit, YB; Lad, NP; Khedkar, VM Synthesis and in vitro evaluations of 6-(hetero)-aryl-imidazo[1,2-b]pyridazine-3-sulfonamide's as an inhibitor of TNF-α production. Bioorg Med Chem Lett 28: 24-30 (2018)
- Akgul, O; Angeli, A; Selleri, S; Capasso, C; Supuran, CT; Carta, F Taurultams incorporating arylsulfonamide: First in vitro inhibition studies of α-, β- and γ-class Carbonic Anhydrases from Vibrio cholerae and Burkholderia pseudomallei. Eur J Med Chem 219: (2021)
- Martínez-Bailén, M; Carmona, AT; Moreno-Clavijo, E; Robina, I; Ide, D; Kato, A; Moreno-Vargas, AJ Tuning of β-glucosidase and α-galactosidase inhibition by generation and in situ screening of a library of pyrrolidine-triazole hybrid molecules. Eur J Med Chem 138: 532-542 (2017)
- Nicholson, JM; Yang, D; Koelblen, T; Hu, EL; Coss, CC; Burris, TP; Hu, X; Micalizio, GC Merging Natural Product Structures with Pharmaceutical Leads: Unnatural Enantiomers of Estranes as Glucocorticoid Receptor Modulators That Suppress TNF-α and IL-6 Release. J Med Chem 67: 16185-16194
- Wang, KM; Ge, YX; Zhang, J; Chen, YT; Zhang, NY; Gu, JS; Fang, L; Zhang, XL; Zhang, J; Jiang, CS New cycloalkyl[b]thiophenylnicotinamide-based α-glucosidase inhibitors as promising anti-diabetic agents: Synthesis, in silico study, in vitro and in vivo evaluations. Bioorg Med Chem Lett 79: (2023)
- Bijian, K; Wernic, D; Nivedha, AK; Su, J; Lim, FPL; Miron, CE; Amzil, H; Moitessier, N; Alaoui-Jamali, MA Novel Aurora A and Protein Kinase C (α, β1, β2, and θ) Multitarget Inhibitors: Impact of Selenium Atoms on the Potency and Selectivity. J Med Chem 65: 3134-3150 (2022)
- Xin, L; Min, J; Hu, H; Li, Y; Du, C; Xie, B; Cheng, Y; Deng, X; Deng, X; Shen, K; Huang, J; Chen, CC; Guo, RT; Dong, C; Zhou, HB Structure-guided identification of novel dual-targeting estrogen receptor α degraders with aromatase inhibitory activity for the treatment of endocrine-resistant breast cancer. Eur J Med Chem 253: (2023)
- Klochkov, VG; Bezsonova, EN; Dubar, M; Melekhina, DD; Temnov, VV; Zaryanova, EV; Lozinskaya, NA; Babkov, DA; Spasov, AA Towards multi-target antidiabetic agents: In vitro and in vivo evaluation of 3,5-disubstituted indolin-2-one derivatives as novel α-glucosidase inhibitors. Bioorg Med Chem Lett 55: (2022)
- Cui, Z; Wang, X; Koppermann, S; Thorson, JS; Ducho, C; Van Lanen, SG Antibacterial Muraymycins from Mutant Strains of Streptomyces sp. NRRL 30471. J Nat Prod 81: 942-948 (2018)
- Hoogenboom, N; Demont, D; de Zwart, E; Verkaik, S; Emmelot, M; van de Kar, B; Kaptein, A; Barf, T Discovery and optimization of covalent EGFR T790M/L858R mutant inhibitors. Bioorg Med Chem Lett 52: (2021)
- Lin, J; Ericsson, A; Campbell, A; Gustafson, G; Wang, Z; Diebold, RB; Ashwell, S; Lancia, Jr., DR; Caravella, JA; Lu, W Fused-bicyclic aryl quinolinone derivatives as mutant-isocitrate dehydrogenase inhibitors US Patent US9624175 (2017)
- LUO, R; LIU, J; HUANG, P; SU, J; FENG, Y; LIU, K; FAN, J; HE, W; QIAN, Y GLUTARIMIDE-CONTAINING PAN-KRAS-MUTANT DEGRADER COMPOUNDS AND USES THEREOF US Patent US20250092039 (2025)
- Okoye-Okafor, UC; Bartholdy, B; Cartier, J; Gao, EN; Pietrak, B; Rendina, AR; Rominger, C; Quinn, C; Smallwood, A; Wiggall, KJ; Reif, AJ; Schmidt, SJ; Qi, H; Zhao, H; Joberty, G; Faelth-Savitski, M; Bantscheff, M; Drewes, G; Duraiswami, C; Brady, P; Groy, A; Narayanagari, SR; Antony-Debre, I; Mitchell, K; Wang, HR; Kao, YR; Christopeit, M; Carvajal, L; Barreyro, L; Paietta, E; Makishima, H; Will, B; Concha, N; Adams, ND; Schwartz, B; McCabe, MT; Maciejewski, J; Verma, A; Steidl, U New IDH1 mutant inhibitors for treatment of acute myeloid leukemia Nat Chem Biol 11: 878-86 (2015)
- Wang, PC; Chiu, DC; Jan, JT; Huang, WI; Tseng, YC; Li, TT; Cheng, TJ; Tsai, KC; Fang, JM Peramivir conjugates as orally available agents against influenza H275Y mutant. Eur J Med Chem 145: 224-234 (2018)
- Heppner, DE; Günther, M; Wittlinger, F; Laufer, SA; Eck, MJ Structural Basis for EGFR Mutant Inhibition by Trisubstituted Imidazole Inhibitors. J Med Chem 63: 4293-4305 (2020)
- Heppner, DE; Wittlinger, F; Beyett, TS; Shaurova, T; Urul, DA; Buckley, B; Pham, CD; Schaeffner, IK; Yang, B; Ogboo, BC; May, EW; Schaefer, EM; Eck, MJ; Laufer, SA; Hershberger, PA Structural Basis for Inhibition of Mutant EGFR with Lazertinib (YH25448). ACS Med Chem Lett 13: 1856-1863 (2022)
- Kargbo, RB Targeting KRAS Mutant Protein Inhibitor for Potential Treatment in Cancer. ACS Med Chem Lett 12: 1633-1634 (2021)
- Kargbo, RB Targeting the KRAS G12D Mutant as Potential Therapy in Cancer. ACS Med Chem Lett 12: 1212-1213 (2021)
- Ohashi, M; Oyama, T; Putranto, EW; Waku, T; Nobusada, H; Kataoka, K; Matsuno, K; Yashiro, M; Morikawa, K; Huh, NH; Miyachi, H Design and synthesis of a series of α-benzyl phenylpropanoic acid-type peroxisome proliferator-activated receptor (PPAR) gamma partial agonists with improved aqueous solubility. Bioorg Med Chem 21: 2319-2332 (2013)
- Haider, S; Alam, MS; Hamid, H; Shafi, S; Nargotra, A; Mahajan, P; Nazreen, S; Kalle, AM; Kharbanda, C; Ali, Y; Alam, A; Panda, AK Synthesis of novel 1,2,3-triazole based benzoxazolinones: their TNF-α based molecular docking with in-vivo anti-inflammatory, antinociceptive activities and ulcerogenic risk evaluation. Eur J Med Chem 70: 579-88 (2013)
- Aldred, GG; Rooney, TPC; Willems, HMG; Boffey, HK; Green, C; Winpenny, D; Skidmore, J; Clarke, JH; Andrews, SP The rational design of ARUK2007145, a dual inhibitor of the α and γ isoforms of the lipid kinase phosphatidylinositol 5-phosphate 4-kinase (PI5P4K). RSC Med Chem 14: 2035-2047 (2023)
- Huang, C; Fischer, C; Machacek, MR; Bogen, S; Biftu, T; Huang, X; Reutershan, MH; Otte, R; Hong, Q; Wu, Z; Yu, Y; Park, M; Chen, L; Biju, P; Knemeyer, I; Lu, P; Kochansky, CJ; Hicks, MB; Liu, Y; Helmy, R; Fradera, X; Donofrio, A; Close, J; Maddess, ML; White, C; Sloman, DL; Sciammetta, N; Lu, J; Gibeau, C; Simov, V; Zhang, H; Fuller, P; Witter, D Diminishing GSH-Adduct Formation of Tricyclic Diazepine-based Mutant IDH1 Inhibitors. ACS Med Chem Lett 13: 734-741 (2022)
- Chan, BK; Hanan, EJ; Bowman, KK; Bryan, MC; Burdick, D; Chan, E; Chen, Y; Clausen, S; Dela Vega, T; Dotson, J; Eigenbrot, C; Elliott, RL; Heald, RA; Jackson, PS; Knight, JD; La, H; Lainchbury, MD; Malek, S; Purkey, HE; Schaefer, G; Schmidt, S; Seward, EM; Sideris, S; Shao, L; Wang, S; Yeap, SK; Yen, I; Yu, C; Heffron, TP Discovery of a Noncovalent, Mutant-Selective Epidermal Growth Factor Receptor Inhibitor. J Med Chem 59: 9080-9093 (2016)
- Cho, H; Shin, I; Ju, E; Choi, S; Hur, W; Kim, H; Hong, E; Kim, ND; Choi, HG; Gray, NS; Sim, T First SAR Study for Overriding NRAS Mutant Driven Acute Myeloid Leukemia. J Med Chem 61: 8353-8373 (2018)
- Wu, F; Jiang, H; Zheng, B; Kogiso, M; Yao, Y; Zhou, C; Li, XN; Song, Y Inhibition of Cancer-Associated Mutant Isocitrate Dehydrogenases by 2-Thiohydantoin Compounds. J Med Chem 58: 6899-908 (2015)
- Kargbo, RB Inhibitors of G12C Mutant Ras Proteins for the Treatment of Cancers. ACS Med Chem Lett 10: 10-11 (2019)
- Kargbo, RB Modulation of KRAS Mutant by Inhibiting PLK1 Kinase in Cancer Therapeutics. ACS Med Chem Lett 12: 1514-1516 (2021)
- Lanman, BA; Wurz, RP; Zhao, W; LI, X; YAMANO, M; HOT, I; RAHIMOFF, R; Pettus, L; Medina, J QUINAZOLINE COMPOUNDS AND USES THEREOF AS INHIBITORS OF MUTANT KRAS PROTEINS US Patent US20250163064 (2025)
- Alpha-Synuclein Competitive Binding Assay (A) Expression and purification of recombinant wild-type human α-synuclein: 0.5 mM IPTG was used to induce production of wild type human α-synuclein by bacteria transformed with a full-length α-synuclein expression plasmid. After shaking at 16° C. for 20 hours, cell pellet was resuspended in lysis buffer (10 mM Tris, 1 mM EGTA, 0.75 mM NaCl, 1 mM PMSF, pH 7.5) and lysed by sonication followed by centrifugation (6000 g, 30 min, 4° C.). The supernatant was then boiled at 95° C. for 15 min with manual agitation by every 5 minutes, followed by centrifugation (6000 g, 30 min, 4° C.). Supernatant was dialyzed with (10 mM Tris, 1 mM EGTA, 50 mM NaCl, pH 7.5) buffer and concentrated with concentrator. The collected sample was loaded onto a Superdex200 column, the flow-through fraction was then loaded onto a Q-HP column. Collected fractions with α-synuclein eluates were pooled, concentrated and stored at −80° C.(B) Preparation of aggregated α-synuclein: 5 mg/mL of α-synuclein in PBS buffer (pH 7.4) was incubated in tube with at 37° C. with shaking (700 rpm) for 5-10 days.(C) In vitro fluorometric α-synuclein binding assays: 2 μM α-synuclein was incubated with serially diluted compound (three-fold serial dilutions, from 10 to 0.001 μM) in a 96-well plate at 37° C. for 1 hour. Fluorescence intensity was read by microplate spectrometer. Compound Kd values were calculated using the following equation: Y=Bmax*X/(Kd+X), where X is the concentration of compound; Y is the fluorescence signal of (compound+α-synuclein)−(compound+DMSO); and Bmax is the maximum signal.
- ChEMBL_2281070 Inhibition of alpha-Synuclein (unknown origin)
- ChEMBL_2295415 Inhibition of Alpha-synuclein (unknown origin) fibrils
- ChEMBL_2277651 Binding affinity to human brain alpha-synuclein fibril
- ChEMBL_2295411 Binding affinity to Alpha-synuclein (unknown origin) assessed as dissociation constant
- ChEMBL_2442952 Binding affinity to alpha-synuclein (unknown origin) assessed as dissociation constant
- ChEMBL_1994333 (CHEMBL4628228) Binding affinity to human wlid-type alpha-synuclein by SPR analysis
- ChEMBL_2295414 Binding affinity to Alpha-synuclein (unknown origin) fibrils assessed as inhibition constant
- ChEMBL_1834277 (CHEMBL4334285) Inhibition of alpha-synuclein (unknown origin) self-aggregation by fluorescence polarization assay
- ChEMBL_2295410 Binding affinity to human recombinant Alpha-synuclein assessed as dissociation constant by fluorometric method
- PBB5 competition assay PBB5 competition assay: 2 μM of α-synuclein was incubated with 0.26 μM 2-(4-(2-(methylamino)pyridine-5-yl)-1,3-butadiene-1-yl)benzothiazole-6-ol (PBB5) and serially diluted compound (three-fold serial dilution, from 10 to 0.001 μM) in a 96-well plate at 37° C. for 1 hour. Fluorescence intensity (excitation/emission=530/690 nm) of PBB5 was read by microplate spectrometer. Percentage of competition was calculated using the following equation:(Max−well of compound alone)/(Max−Min)*100%, where Max=(α-synuclein+PBB5) signals−(buffer+PBB5) signals; and Min=(buffer+PBB5) signals−(buffer+PBB5) signals.
- ChEMBL_2315362 Binding affinity to biotinylated recombinant alpha-synuclein (unknown origin) assessed as dissociation constant by BLI assay
- ChEMBL_2320669 Inhibition of alpha-Synuclein (unknown origin) aggregation incubated for 72 hrs by ThT-based fluorescence analysis
- ChEMBL_2440499 Binding affinity to Alpha-synuclein (unknown origin) preformed fibrils assessed as dissociation constant by FP assay
- ChEMBL_2468944 Induction of his-tagged human alpha-Synuclein fibril depolymerization measured for 1 day by ThT fluorescence assay
- ChEMBL_405876 (CHEMBL909175) Inhibition of mutant HIV1 integrase C130A mutant 3' processing step
- ChEMBL_1991810 (CHEMBL4625545) Inhibition of alpha-synuclein (unknown origin) aggregation incubated for 3 days by thioflavin T based fluorescence assay
- ChEMBL_2045851 (CHEMBL4700550) Inhibition of alpha-synuclein aggregation (unknown origin) incubated for 8 days by thioflavin S based fluorescence assay
- ChEMBL_2045872 (CHEMBL4700571) Binding affinity to alpha-synuclein (unknown origin) expressed in Escherichia coli BL21(DE3) pLysS by fluorescence method
- ChEMBL_2045873 (CHEMBL4700572) Binding affinity to alpha-synuclein (unknown origin) expressed in Escherichia coli BL21(DE3) by circular dichroism analysis
- ChEMBL_2295413 Binding affinity to human recombinant Alpha-synuclein expressed in Escherichia coli assessed as dissociation constant at 200 uM
- ChEMBL_2448617 Inhibition of wild type full length recombinant alpha-synuclein (unknown origin) expressed in Escherichia coli by spectrophotometric analysis
- ChEMBL_1505263 (CHEMBL3594888) Binding affinity to recombinant alpha-synuclein (unknown origin) expressed in Escherichia coli after 1 hr by scintillation counting
- ChEMBL_2045854 (CHEMBL4700553) Inhibition of alpha-synuclein fibril formation (unknown origin) incubated for 6 days by thioflavin S based fluorescence assay
- ChEMBL_2045865 (CHEMBL4700564) Binding affinity to human alpha-synuclein (1 to 140 residues) expressed in Escherichia coli by isothermal titration calorimetry
- ChEMBL_2328649 Inhibition of recombinant human Alpha-synuclein aggregation expressed in Escherichia coli incubated for 72 hrs by ThT fluorescence assay
- ChEMBL_1505256 (CHEMBL3594881) Displacement of Thio-T from recombinant alpha-synuclein (unknown origin) expressed in Escherichia coli after 1.5 hrs by fluorescence assay
- ChEMBL_2479224 Inhibition of alpha-Synuclein (unknown origin) aggregation assessed as aggregation inhibition ratio incubated for 72 hrs by Thioflavin T fluorescence assay
- ChEMBL_2045853 (CHEMBL4700552) Inhibition of alpha-synuclein fibril formation (unknown origin) incubated for 24 hrs to 7 days by thioflavin S based fluorescence assay
- ChEMBL_2045866 (CHEMBL4700565) Inhibition of wild type human alpha-synuclein fibrillization expressed in Escherichia coli BL21(DE3)pLysS by thioflavin-T based fluorescence assay
- ChEMBL_2475603 Inhibition of alpha-Synuclein (unknown origin) aggregation assessed as aggregation inhibition ratio measured after 72 hrs by Thioflavin T based fluorescence assay
- ChEMBL_831727 (CHEMBL2065090) Binding affinity to human alpha-synuclein expressed in Escherichia coli BL21 (DE3) cells after 1 hr by thioflavin T fluorescence assay
- In Vitro Inhibition Assay α-Synucleinopathies are characterised by aggregation of α-synuclein in neurons. Aggregation of purified α-synuclein is performed essentially as described by Gerard et al. FASEB. 20(3):524-6 (2006). 20-100 μg purified α-synuclein (Sigma; S7820) at a concentration of about 2.5 μg/mL is incubated in the presence of spermin (250 μM) or paraquat (32 mM) or 6-hydroxydopamine (400 μM) or vehicle in a 384 well plate. Spermin, paraquat and 6-hydroxydopamine promote the α-synuclein aggregation process. Aggregation kinetics is determined by measuring turbidity at 340 nm, every 1-15 minutes for at least one hour.
- ChEMBL_2264767 Inhibition of FLT3 mutant (unknown origin)
- ChEMBL_2265574 Inhibition of mutant HTT (unknown origin)
- ChEMBL_2278144 Inhibition of human RET V804L mutant
- ChEMBL_2278145 Inhibition of human RET V804M mutant
- ChEMBL_2280927 Inhibition of human BRAF V600E mutant
- ChEMBL_2360427 Inhibition of human LRRK2 G2019S mutant
- ChEMBL_2360428 Inhibition of human LRRK2 I2020T mutant
- ChEMBL_2467729 Inhibition of human IDH1 R132H mutant
- ChEMBL_2495607 Inhibition of human IDH1 R132H mutant
- ChEMBL_382857 (CHEMBL869786) Inhibition of PTP1B V49A mutant
- ChEMBL_382858 (CHEMBL869785) Inhibition of PTP1B I219A mutant
- ChEMBL_382864 (CHEMBL869792) Inhibition of TCPTP I220A mutant
- ChEMBL_424220 (CHEMBL908994) Inhibition of Abl T315I mutant
- ChEMBL_459603 (CHEMBL925703) Inhibition of HIV1 protease mutant
- ChEMBL_501661 (CHEMBL981409) Inhibition of Abl T315I mutant
- ChEMBL_523244 (CHEMBL1000620) Inhibition of BRAF V600E mutant
- ChEMBL_617631 (CHEMBL1101240) Inhibition of BRAF V600E mutant
- ChEMBL_831688 (CHEMBL2065051) Inhibition of BRAF V600E mutant
- ChEMBL_1842065 (CHEMBL4342364) Inhibition of alpha-synuclein aggregation (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 3 days by thioflavin T fluorescence assay
- ChEMBL_2045858 (CHEMBL4700557) Binding affinity to alpha-synuclein oligomer (unknown origin) expressed in Escherichia coli BL21(DE3) cells incubated for 30 mins by spectrofluorometric analysis
- ChEMBL_2443862 Potentiation of CFTR N1303K mutant (unknown origin) expressed in FRT cells assessed as increase in CFTR mutant activity
- ChEMBL_2045857 (CHEMBL4700556) Binding affinity to alpha-synuclein preformed fibrils (unknown origin) expressed in Escherichia coli BL21(DE3) cells incubated for 30 mins by spectrofluorometric analysis
- ChEMBL_2045874 (CHEMBL4700573) Binding affinity to human alpha-synuclein (1-140 residues) expressed in Escherichia coli BL21(DE3) assessed as first dissociation constant by mass-spectrometry
- ChEMBL_2045875 (CHEMBL4700574) Binding affinity to human alpha-synuclein (1-140 residues) expressed in Escherichia coli BL21(DE3) assessed as second dissociation constant by mass-spectrometry
- ChEMBL_2379496 Competitive binding affinity to recombinant alpha-synuclein (unknown origin) assessed as inhibition constant incubated for 0.5 hrs by ThT-based fluorescence microplate reader analysis
- ChEMBL_971423 (CHEMBL2406136) Inhibition of Plk2 (unknown origin) transfected in HEK293 cells assessed as inhibition of alpha-synuclein phosphorylation at ser129 after 2 hrs by ELISA
- ChEMBL_1476345 (CHEMBL3429868) Inhibition of human Src T341M mutant
- ChEMBL_1794140 (CHEMBL4266257) Inhibition of L1196M mutant (unknown origin)
- ChEMBL_1822317 (CHEMBL4322081) Inhibition of HIV1 protease V82F mutant
- ChEMBL_1822318 (CHEMBL4322082) Inhibition of HIV1 protease G48V mutant
- ChEMBL_1822319 (CHEMBL4322083) Inhibition of HIV1 protease V82A mutant
- ChEMBL_1933800 (CHEMBL4479452) Inhibition of human EGFR T790M mutant
- ChEMBL_2050820 (CHEMBL4705519) Inhibition of human ALK C1156Y mutant
- ChEMBL_2050821 (CHEMBL4705520) Inhibition of human ALK L1196M mutant
- ChEMBL_2074230 (CHEMBL4729764) Inhibition of human FBPase S124A mutant
- ChEMBL_2074231 (CHEMBL4729765) Inhibition of human FBPase N125A mutant
- ChEMBL_2074232 (CHEMBL4729766) Inhibition of human FBPase D127A mutant
- ChEMBL_2074233 (CHEMBL4729767) Inhibition of human FBPase R243A mutant
- ChEMBL_2074235 (CHEMBL4729769) Inhibition of human FBPase Y258A mutant
- ChEMBL_2110583 (CHEMBL4819433) Inhibition of mouse COX2 V523I mutant
- ChEMBL_2110584 (CHEMBL4819434) Inhibition of mouse COX2 S530A mutant
- ChEMBL_2110585 (CHEMBL4819435) Inhibition of mouse COX2 R120Q mutant
- ChEMBL_2264768 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2265309 Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2270533 Inhibition of FLT3-ITD mutant (unknown origin)
- ChEMBL_2270534 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2270536 Inhibition of FLT3 D835H mutant (unknown origin)
- ChEMBL_2270572 Inhibition of FLT3-D835Y mutant (unknown origin)
- ChEMBL_2271436 Inhibition of Abl T315I mutant (unknown origin)
- ChEMBL_2277133 Inhibition of HIV-1 integrase Q148K mutant
- ChEMBL_2278125 Inhibition of KIT T670I mutant (unknown origin)
- ChEMBL_2278126 Inhibition of KIT D816V mutant (unknown origin)
- ChEMBL_2278127 Inhibition of PDGFRalpha D842V mutant (unknown origin)
- ChEMBL_2278133 Inhibition of FGFR1 V561M mutant (unknown origin)
- ChEMBL_2278134 Inhibition of FGFR2 V564M mutant (unknown origin)
- ChEMBL_2278135 Inhibition of FGFR3 V555M mutant (unknown origin)
- ChEMBL_2278137 Inhibition of FGFR4 V550L mutant (unknown origin)
- ChEMBL_2278142 Inhibition of RET V804M mutant (unknown origin)
- ChEMBL_2278146 Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2278147 Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2281215 Inhibition of SHP2 (unknown origin) E76K mutant
- ChEMBL_2283198 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2284321 Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2290578 Inhibition of FGFR3 V555M mutant (unknown origin)
- ChEMBL_2294502 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2298700 Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_2299115 Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2321730 Inhibition of CFTR G551D mutant (unknown origin)
- ChEMBL_2321782 Inhibition of TTR V30M mutant (unknown origin)
- ChEMBL_2321783 Inhibition of TTR V122I mutant (unknown origin)
- ChEMBL_2327410 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2331104 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2331105 Inhibition of EGFR Del19 mutant (unknown origin)
- ChEMBL_2331106 Inhibition of EGFR LTb mutant (unknown origin)
- ChEMBL_2331107 Inhibition of EGFR dTCb mutant (unknown origin)
- ChEMBL_2331108 Inhibition of EGFR LTCb mutant (unknown origin)
- ChEMBL_2336380 Inhibition of JAK2 V617F mutant (unknown origin)
- ChEMBL_2342478 Inhibition of LRRK2 G2019S mutant (unknown origin)
- ChEMBL_2346395 Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_2346396 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2348662 Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2348663 Inhibition of ALK G1269A mutant (unknown origin)
- ChEMBL_2348664 Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2353757 Inhibition of NSD2 E1099K mutant (unknown origin)
- ChEMBL_2353758 Inhibition of NSD2 T1150A mutant (unknown origin)
- ChEMBL_2356567 Inhibition of KIT D816V mutant (unknown origin)
- ChEMBL_2356568 Inhibition of PDGFRalpha D842V mutant (unknown origin)
- ChEMBL_2357239 Inhibition of IDH2 R140Q mutant (unknown origin)
- ChEMBL_2359390 Inhibition of EZH2 Y641F mutant (unknown origin)
- ChEMBL_2360390 Inhibition of LRRK2 G2019S mutant (unknown origin)
- ChEMBL_2368851 Inhibition of TRKA G667C mutant (unknown origin)
- ChEMBL_2377742 Inhibition of PI3Kalpha H1047R mutant (unknown origin)
- ChEMBL_2379324 Inhibition of HIV-1 integrase Q148K mutant
- ChEMBL_2379384 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2424714 Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2427193 Inhibition of JAK2 V617F mutant (unknown origin)
- ChEMBL_2427237 Inhibition of FGFR4 C552A mutant (unknown origin)
- ChEMBL_2427238 Inhibition of FGFR4 C477A mutant (unknown origin)
- ChEMBL_2434882 Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_2438537 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2443858 Potentiation of CFTR F508del mutant (unknown origin)
- ChEMBL_2443866 Activation of CFTR F508del mutant (unknown origin)
- ChEMBL_2444527 Agonist activity against rat mGlu4 R60A mutant
- ChEMBL_2444528 Agonist activity against rat mGlu4 K74A mutant
- ChEMBL_2444529 Agonist activity against rat mGlu4 S160A mutant
- ChEMBL_2444530 Agonist activity against rat mGlu4 S160D mutant
- ChEMBL_2444531 Agonist activity at rat mGlu4 R258A mutant
- ChEMBL_2444535 Agonist activity at rat mGlu7 K60A mutant
- ChEMBL_2444537 Agonist activity at rat mGlu7 N74A mutant
- ChEMBL_2444538 Agonist activity at rat mGlu7 N74K mutant
- ChEMBL_2444539 Agonist activity at rat mGlu7 S160A mutant
- ChEMBL_2444540 Agonist activity at rat mGlu7 Q258A mutant
- ChEMBL_2451391 Inhibition of RET V804M mutant (unknown origin)
- ChEMBL_2451404 Inhibition of RET V804L mutant (unknown origin)
- ChEMBL_2451407 Inhibition of RET G810A mutant (unknown origin)
- ChEMBL_2451408 Inhibition of RET G810D mutant (unknown origin)
- ChEMBL_2451409 Inhibition of RET G810S mutant (unknown origin)
- ChEMBL_2451410 Inhibition of RET G810V mutant (unknown origin)
- ChEMBL_2451411 Inhibition of RET G810C mutant (unknown origin)
- ChEMBL_2451412 Inhibition of RET G810R mutant (unknown origin)
- ChEMBL_2459012 Inhibition of EZH2 Y641F mutant (unknown origin)
- ChEMBL_2470558 Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2476258 Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2476282 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2476909 Inhibition of FGFR2 V564F mutant (unknown origin)
- ChEMBL_2481991 Inhibition of TRKA G595R mutant (unknown origin)
- ChEMBL_2481992 Inhibition of TRKA G667C mutant (unknown origin)
- ChEMBL_2481998 Inhibition of TRKC G623R mutant (unknown origin)
- ChEMBL_2481999 Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2482001 Inhibition of RET G810R mutant (unknown origin)
- ChEMBL_2482003 Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_2482004 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2484874 Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_2484875 Inhibition of IDH1 R132C mutant (unknown origin)
- ChEMBL_2488198 Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2488975 Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_2488979 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_2489716 Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2489717 Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2491809 Inhibition of FLT3 D835H mutant (unknown origin)
- ChEMBL_2492004 Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2495358 Inhibition of FLT3-ITD mutant (unknown origin)
- ChEMBL_2495629 Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_2495630 Inhibition of IDH1 R132C mutant (unknown origin)
- ChEMBL_2495631 Inhibition of IDH2 R140Q mutant (unknown origin)
- ChEMBL_2495632 Inhibition of IDH2 R172K mutant (unknown origin)
- ChEMBL_2497249 Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2500676 Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_2501362 Inhibition of KIT D816V mutant (unknown origin)
- ChEMBL_2504395 Inhibition of EZH2 Y641F mutant (unknown origin)
- ChEMBL_2511011 Inhibition of Abl T315I mutant (unknown origin)
- ChEMBL_2516364 Inhibition of RET V804L mutant (unknown origin)
- ChEMBL_2516365 Inhibition of RET Y791F mutant (unknown origin)
- ChEMBL_2584006 Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_391664 (CHEMBL871553) Inhibition of human ACE Y1096F mutant
- ChEMBL_391665 (CHEMBL871554) Inhibition of human ACE K1087A mutant
- ChEMBL_399772 (CHEMBL910307) Inhibition of HIV1 protease I8 mutant
- ChEMBL_445721 (CHEMBL896012) Inhibition of HIV1 protease D30N mutant
- ChEMBL_445722 (CHEMBL896013) Inhibition of HIV1 protease I50V mutant
- ChEMBL_445723 (CHEMBL896014) Inhibition of HIV1 protease V82A mutant
- ChEMBL_445724 (CHEMBL896015) Inhibition of HIV1 protease I84V mutant
- ChEMBL_491409 (CHEMBL945372) Inhibition of HTLV1 protease L40I mutant
- ChEMBL_646978 (CHEMBL1217119) Inhibition of B-Raf V600E mutant
- ChEMBL_766930 (CHEMBL1828155) Binding affinity to BRAF V600E mutant
- ChEMBL_1781004 (CHEMBL4252521) Inhibition of wild type alpha-synuclein (unknown origin) aggregation expressed in Escherichia coli BL21 after 30 days by thioflavin T staining-based spectrofluorimetric analysis
- ChEMBL_2045852 (CHEMBL4700551) Inhibition of human alpha-synuclein filament formation expressed in Escherichia coli BL21(DE3) cells incubated for 72 hrs by thioflavin S based fluorescence assay
- ChEMBL_2045859 (CHEMBL4700558) Binding affinity to alpha-synuclein LMV 100 kDa (unknown origin) expressed in Escherichia coli BL21(DE3) cells incubated for 30 mins by spectrofluorometric analysis
- ChEMBL_2045860 (CHEMBL4700559) Binding affinity to alpha-synuclein LMV 50 kDa (unknown origin) expressed in Escherichia coli BL21(DE3) cells incubated for 30 mins by spectrofluorometric analysis
- Cell-Based Phosho-ERK Assay Mutant BRAF WM266.4 Cells.
- ChEMBL_1277030 (CHEMBL3088499) Inhibition of EGFR L851Q mutant (unknown origin)
- ChEMBL_1277031 (CHEMBL3088500) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_1277032 (CHEMBL3088501) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_1435523 (CHEMBL3385343) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1436082 (CHEMBL3384713) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1452616 (CHEMBL3363741) Inhibition of BRAF V599E mutant (unknown origin)
- ChEMBL_1460931 (CHEMBL3396751) Inhibition of LRRK2 G2019S mutant (unknown origin)
- ChEMBL_1476349 (CHEMBL3429872) Inhibition of human B-RAF V600E mutant
- ChEMBL_1477235 (CHEMBL3428927) Inhibition of RET (unknown origin) V804 mutant
- ChEMBL_1498377 (CHEMBL3584206) Inhibition of PI3Kalpha H1047R mutant (unknown origin)
- ChEMBL_1502545 (CHEMBL3592570) Inhibition of LRRK2 G2019S mutant (unknown origin)
- ChEMBL_1544406 (CHEMBL3749784) Inhibition of FGFR3 K650E mutant (unknown origin)
- ChEMBL_1551286 (CHEMBL3762137) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_1555117 (CHEMBL3768039) Inhibition of BRAF V599E mutant (unknown origin)
- ChEMBL_1711186 (CHEMBL4121235) Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_1714378 (CHEMBL4124427) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_1732047 (CHEMBL4147583) Inhibition of Mycobacterium tuberculosis InhA T2A mutant
- ChEMBL_1732049 (CHEMBL4147585) Inhibition of Mycobacterium tuberculosis InhA D2A mutant
- ChEMBL_1732050 (CHEMBL4147586) Inhibition of Mycobacterium tuberculosis InhA S94A mutant
- ChEMBL_1744973 (CHEMBL4179483) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_1746180 (CHEMBL4180690) Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_1746385 (CHEMBL4180895) Inhibition of IDH1 R132C mutant (unknown origin)
- ChEMBL_1746388 (CHEMBL4180898) Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_1749937 (CHEMBL4184697) Inhibition of serine racemase mutant (unknown origin)
- ChEMBL_1753120 (CHEMBL4187880) Inhibition of human BCR/ABL V299L mutant
- ChEMBL_1759736 (CHEMBL4194744) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1764602 (CHEMBL4199849) Inhibition of p110alpha H1047R mutant (unknown origin)
- ChEMBL_1784722 (CHEMBL4256239) Inhibition of IDH1 R132G mutant (unknown origin)
- ChEMBL_1784723 (CHEMBL4256240) Inhibition of IDH1 R132L mutant (unknown origin)
- ChEMBL_1784724 (CHEMBL4256241) Inhibition of IDH1 R132S mutant (unknown origin)
- ChEMBL_1794128 (CHEMBL4266245) Inhibition of ROS1 L2026M mutant (unknown origin)
- ChEMBL_1794129 (CHEMBL4266246) Inhibition of ROS1 G2032R mutant (unknown origin)
- ChEMBL_1803655 (CHEMBL4275947) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1811382 (CHEMBL4310842) Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_1815502 (CHEMBL4315076) Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_1815509 (CHEMBL4315083) Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_1825952 (CHEMBL4325716) Inhibition of IDH2 R172K mutant (unknown origin)
- ChEMBL_1825953 (CHEMBL4325717) Inhibition of IDH2 R140Q mutant (unknown origin)
- ChEMBL_1830190 (CHEMBL4330198) Inhibition of ROS1 G2032R mutant (unknown origin)
- ChEMBL_1830191 (CHEMBL4330199) Inhibition of TRKA G595R mutant (unknown origin)
- ChEMBL_1833686 (CHEMBL4333694) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1842485 (CHEMBL4342912) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_1854855 (CHEMBL4355584) Binding affinity to human FLT3 F691L mutant
- ChEMBL_1859457 (CHEMBL4360313) Inhibition of IDH2 R172K mutant (unknown origin)
- ChEMBL_1859458 (CHEMBL4360314) Inhibition of IDH2 R140Q mutant (unknown origin)
- ChEMBL_1859776 (CHEMBL4360632) Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_1859777 (CHEMBL4360633) Inhibition of FLT3 D835Y mutant (unknown origin)
- ChEMBL_1859778 (CHEMBL4360634) Inhibition of FLT3 D835H mutant (unknown origin)
- ChEMBL_1863470 (CHEMBL4364445) Inhibition of ABL Q252H mutant (unknown origin)
- ChEMBL_1863471 (CHEMBL4364446) Inhibition of ABL Y253F mutant (unknown origin)
- ChEMBL_1863509 (CHEMBL4364484) Inhibition of ABL T315I mutant (unknown origin)
- ChEMBL_1873927 (CHEMBL4375216) Inhibition of BTK Cys481S mutant (unknown origin)
- ChEMBL_1886827 (CHEMBL4388504) Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_1886828 (CHEMBL4388505) Inhibition of ALK F1174L mutant (unknown origin)
- ChEMBL_1898989 (CHEMBL4401104) Inhibition of PTP1B A27S mutant (unknown origin)
- ChEMBL_1901573 (CHEMBL4403795) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_1904563 (CHEMBL4406785) Inhibition of ALK2 G328V mutant (unknown origin)
- ChEMBL_1904565 (CHEMBL4406787) Inhibition of ALK2 R258G mutant (unknown origin)
- ChEMBL_1906420 (CHEMBL4408778) Binding affinity to human FLT3 D385V mutant
- ChEMBL_1906421 (CHEMBL4408779) Binding affinity to human FLT3 ITD mutant
- ChEMBL_195344 (CHEMBL802586) Inhibitory activity against E138K mutant reverse transcriptase
- ChEMBL_195380 (CHEMBL802417) Inhibitory activity against R172A mutant reverse transcriptase
- ChEMBL_1978042 (CHEMBL4611177) Inhibition of BTK C481S mutant (unknown origin)
- ChEMBL_1994767 (CHEMBL4628662) Inhibition of IDH2 R140Q mutant (unknown origin)
- ChEMBL_1994769 (CHEMBL4628664) Inhibition of IDH1 R132C mutant (unknown origin)
- ChEMBL_1994770 (CHEMBL4628665) Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_1994772 (CHEMBL4628667) Inhibition of IDH2 R172K mutant (unknown origin)
- ChEMBL_2014754 (CHEMBL4668332) Inhibition of LRRK2 G2019S mutant (unknown origin)
- ChEMBL_2017084 (CHEMBL4670662) Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_2020938 (CHEMBL4674751) Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2059833 (CHEMBL4714834) Inhibition of FGFR4 C552A mutant (unknown origin)
- ChEMBL_2068038 (CHEMBL4723291) Inhibition of FGFR4 C552A mutant (unknown origin)
- ChEMBL_2069117 (CHEMBL4724370) Inhibition of SRC S345C mutant (unknown origin)
- ChEMBL_2069124 (CHEMBL4724377) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2072507 (CHEMBL4728041) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2074184 (CHEMBL4729718) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2076906 (CHEMBL4732697) Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_2081534 (CHEMBL4737325) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2082527 (CHEMBL4738318) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2108731 (CHEMBL4817406) Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2119800 (CHEMBL4828947) Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2119801 (CHEMBL4828948) Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2127108 (CHEMBL4836453) Inhibition of RET V804M mutant (unknown origin)
- ChEMBL_2127109 (CHEMBL4836454) Inhibition of RET M918T mutant (unknown origin)
- ChEMBL_2132161 (CHEMBL4841676) Inhibition of BTK C481S mutant (unknown origin)
- ChEMBL_2135214 (CHEMBL4844824) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2147692 (CHEMBL5032038) Inhibition of TRKA G595R mutant (unknown origin)
- ChEMBL_2150708 (CHEMBL5035170) Inhibition of TrKA G595R mutant (unknown origin)
- ChEMBL_2150709 (CHEMBL5035171) Inhibition of TrKA G667C mutant (unknown origin)
- ChEMBL_2157425 (CHEMBL5042085) Inhibition of FLT3-ITD mutant (unknown origin)
- ChEMBL_2162453 (CHEMBL5047314) Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_2171652 (CHEMBL5056786) Inhibition of BTK C481S mutant (unknown origin)
- ChEMBL_2184494 (CHEMBL5096576) Inhibition of ALK L1196M mutant (unknown origin)
- ChEMBL_2184495 (CHEMBL5096577) Inhibition of ALK G1202R mutant (unknown origin)
- ChEMBL_2185635 (CHEMBL5097717) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2185653 (CHEMBL5097735) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2185696 (CHEMBL5097778) Inhibition of BRAF V599E mutant(unknown origin)
- ChEMBL_2196432 (CHEMBL5108948) Inhibition of Abl T315I mutant (unknown origin)
- ChEMBL_2201428 (CHEMBL5114136) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2201792 (CHEMBL5114500) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2201793 (CHEMBL5114501) Inhibition of EGFR del19 mutant (unknown origin)
- ChEMBL_2203820 (CHEMBL5116528) Agonist activity at human STING HAQ mutant
- ChEMBL_2208442 (CHEMBL5121391) Inhibition of EZH2 Y641N mutant (unknown origin)
- ChEMBL_2208451 (CHEMBL5121400) Inhibition of IDH1 R132H mutant (unknown origin)
- ChEMBL_2210982 (CHEMBL5123931) Inhibition of EZH2 Y641F mutant (unknown origin)
- ChEMBL_2210986 (CHEMBL5123935) Inhibition of EZH2 Y641N mutant (unknown origin)
- ChEMBL_2211441 (CHEMBL5124390) Inhibition of BTK C481S mutant (unknown origin)
- ChEMBL_2213961 (CHEMBL5127093) Inhibition of EGFR T790M mutant (unknown origin)
- ChEMBL_2214133 (CHEMBL5127265) Inhibition of BTK C481S mutant (unknown origin)
- ChEMBL_2221627 (CHEMBL5134961) Inhibition of FLT3 ITD mutant (unknown origin)
- ChEMBL_2224731 (CHEMBL5138244) Inhibition of EZH2 Y641N mutant (unknown origin)
- ChEMBL_2226361 (CHEMBL5139874) Inhibition of TRKA G595R mutant (unknown origin)
- ChEMBL_2228893 (CHEMBL5142406) Inhibition of ABL1 T315I mutant (unknown origin)
- ChEMBL_2230005 (CHEMBL5143777) Inhibition of EGFR L858R mutant (unknown origin)
- ChEMBL_2232293 (CHEMBL5146065) Inhibition of TRKA G595R mutant (unknown origin)
- ChEMBL_2236238 (CHEMBL5150134) Inhibition of KRAS G12C mutant (unknown origin)
- ChEMBL_2260100 (CHEMBL5215111) Inhibition of BRAF V600E mutant (unknown origin)
- ChEMBL_2271111 Inhibition of B-Raf V600E mutant (unknown origin)
- ChEMBL_2271480 Potentiation of CFTR F508del mutant activity (unknown origin)
- ChEMBL_2278123 Inhibition of Bcr-Abl T315I mutant (unknown origin)
- ChEMBL_2281216 Inhibition of SHP2 T253M/Q257L mutant (unknown origin)
- ChEMBL_2281217 Inhibition of SHP2 1-525 mutant (unknown origin)
- ChEMBL_2283196 Inhibition of EGFR T790M/C797S mutant (unknown origin)
- ChEMBL_2283199 Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2321729 Inhibition of CFTR F508 deletion mutant (unknown origin)
- ChEMBL_2339759 Binding affinity to ERalpha Y537S mutant (unknown origin)
- ChEMBL_2342480 Inhibition of LRRK2 G2019S mutant in HEK293 cells
- ChEMBL_2347660 Inhibition of EGFR T790M/L858R mutant (unknown origin)
- ChEMBL_2359676 Binding affinity to FLT3 D835H mutant (unknown origin)
- ChEMBL_2359694 Binding affinity to FLT3 D835V mutant (unknown origin)
- ChEMBL_2359695 Binding affinity to FLT3 D835Y mutant (unknown origin)
- ChEMBL_2359696 Binding affinity to FLT3 ITD mutant (unknown origin)
- ChEMBL_2359699 Binding affinity to FLT3 K663Q mutant (unknown origin)
- ChEMBL_2359700 Binding affinity to FLT3 N841I mutant (unknown origin)
- ChEMBL_2359701 Binding affinity to FLT3 R834Q mutant (unknown origin)
- ChEMBL_2444536 Agonist activity at rat mGlu7 K60A/S160A mutant
- ChEMBL_2458802 Inhibition of SOS1/KRAS G12C mutant (unknown origin)
- ChEMBL_2460398 Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2472959 Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2476958 Inhibition of EGFR T790M/L858R mutant (unknown origin)
- ChEMBL_2477216 Inhibition of B-RAF V600E mutant (unknown origin)
- ChEMBL_2479453 Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2481988 Inhibition of EML4-ALK G1202R mutant (unknown origin)
- ChEMBL_2490917 Inhibition of B-Raf V600E mutant (unknown origin)
- ChEMBL_2495621 Inhibition of wildtype IDH2 R172K mutant (unknown origin)
- ChEMBL_2497252 Inhibition of B-RAF V600E mutant (unknown origin)
- ChEMBL_2542158 Inhibition of B-Raf V600E mutant (unknown origin)
- ChEMBL_302213 (CHEMBL826986) Dissociation constant against T4 lysozyme mutant L99A
- ChEMBL_402353 (CHEMBL866167) Binding affinity to ERalpha L384M/M421G mutant
- ChEMBL_450286 (CHEMBL900567) Inhibition of HIV1 reverse transcriptase K103N mutant
- ChEMBL_450287 (CHEMBL900568) Inhibition of HIV1 reverse transcriptase L100I mutant
- ChEMBL_450288 (CHEMBL900569) Inhibition of HIV1 reverse transcriptase V106A mutant
- ChEMBL_450289 (CHEMBL900570) Inhibition of HIV1 reverse transcriptase V179D mutant
- ChEMBL_450291 (CHEMBL900572) Inhibition of HIV1 reverse transcriptase Y188L mutant
- ChEMBL_478366 (CHEMBL931318) Inhibition of Rhizobium leguminosarum LpxC S214G mutant
- ChEMBL_553815 (CHEMBL966178) Inhibition of recombinant B-RAF-V600E mutant
- ChEMBL_574942 (CHEMBL1026222) Inhibition of recombinant B-RAF-V600E mutant
- ChEMBL_598996 (CHEMBL1041920) Inhibition of HIV1 HXB2 protease L90M mutant
- ChEMBL_601567 (CHEMBL1047747) Inhibition of HIV1 reverse transcriptase Y181C mutant
- ChEMBL_601568 (CHEMBL1047748) Inhibition of HIV1 reverse transcriptase K103N mutant
- ChEMBL_643503 (CHEMBL1212367) Inhibition of HCV NS5B polymerase L419M mutant
- ChEMBL_643504 (CHEMBL1212368) Inhibition of HCV NS5B polymerase M414T mutant
- ChEMBL_675253 (CHEMBL1273235) Inhibition of HIV1 reverse transcriptase L100I mutant
- ChEMBL_675254 (CHEMBL1273236) Inhibition of HIV1 reverse transcriptase V106A mutant
- ChEMBL_675255 (CHEMBL1273237) Inhibition of HIV1 reverse transcriptase Y188L mutant
- ChEMBL_712673 (CHEMBL1660416) Inhibition of HIV1 Reverse transcriptase P119S mutant
- ChEMBL_712674 (CHEMBL1660417) Inhibition of HIV1 Reverse transcriptase T165A mutant
- ChEMBL_712675 (CHEMBL1660418) Inhibition of HIV1 Reverse transcriptase M184V mutant
- ChEMBL_756683 (CHEMBL1805148) Inhibition of HIV1 reverse transcriptase K103N mutant
- ChEMBL_756684 (CHEMBL1805149) Inhibition of HIV1 reverse transcriptase L100I mutant
- ChEMBL_756685 (CHEMBL1805150) Inhibition of HIV1 reverse transcriptase V179D mutant
- ChEMBL_835066 (CHEMBL2073610) Inhibition of BRAF V600E mutant by ELISA
- ChEMBL_2045855 (CHEMBL4700554) Inhibition of wild type alpha-synuclein aggregation (unknown origin) expressed in Escherichia coli BL21 cells incubated for 30 days by thioflavin T based fluorescence assay
- ChEMBL_2295416 Binding affinity to C-terminal His6-tagged human Alpha-synuclein fibrils (1 to 97 residues) expressed in Escherichia coli assessed as dissociation constant by chromatography method
- ChEMBL_2428851 Inhibition of alpha-synuclein (unknown origin) aggregation inhibition ratio by measuring relative fluorescence intensity measured after 3 days by ThT F1 based assay relative to control
- Competitive Binding Assay The compounds prepared in the preceding examples were found to bind with high affinity to α-synuclein aggregates/fibrils that are found in the hallmark Lewy Bodies of Parkinson's disease. In order to assess relative binding affinities of the test compounds for aggregated α-synuclein, competition assays were set up with a radiolabeled molecule already known to bind to α-synuclein fibrils and non-radiolabeled test compounds. In order to induce its aggregation, α-synuclein was incubated in phosphate buffered saline (PBS, pH 7.4) at 37° C. for three days with shaking (1,400 rpm). Competitive binding assays were carried out in 12×75 mm borosilicate glass tubes. The reaction mixture contained 100 μL of α-synuclein aggregates (0.5-1 μg), [3H] positive reference compound #1 (100-200 nM diluted in PBS) and 50 μL of competing compounds (10−5-10−9 M, diluted serially in PBS containing 0.1% bovine serum albumin) in a final volume of 0.25 ml.
- ChEMBL_2211422 (CHEMBL5124371) Inhibition of alpha-Synuclein (unknown origin) aggregation assessed as aggregation inhibitory ratio by measuring relative fluorescence intensity and measured after 72 hrs by ThT flourescence assay
- ChEMBL_1444339 (CHEMBL3371770) Inhibition of B-Raf V600E mutant (unknown origin)
- ChEMBL_1459997 (CHEMBL3368317) Inhibition of c-KIT D816V mutant (unknown origin)
- ChEMBL_1471572 (CHEMBL3420307) Inhibition of EGFR T790M/L858R mutant (unknown origin)
- ChEMBL_1517141 (CHEMBL3619924) Inhibition of c-Src E280G mutant (unknown origin)
- ChEMBL_1526213 (CHEMBL3636898) Inhibition of B-RAF V600E mutant (unknown origin)
- ChEMBL_1551276 (CHEMBL3762127) Inhibition of EGFR T790M/L858R mutant (unknown origin)
- ChEMBL_1738283 (CHEMBL4154033) Binding affinity to p53 Y220C mutant (unknown origin)
- ChEMBL_1744974 (CHEMBL4179484) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_1751517 (CHEMBL4186277) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_1759924 (CHEMBL4194932) Binding affinity to KIT D816V mutant (unknown origin)
- ChEMBL_1759925 (CHEMBL4194933) Binding affinity to KIT V816H mutant (unknown origin)
- ChEMBL_1759926 (CHEMBL4194934) Binding affinity to KIT V559D mutant (unknown origin)
- ChEMBL_1795809 (CHEMBL4267926) Inhibition of recombinant HIV1 reverse transcriptase L100I mutant
- ChEMBL_1795810 (CHEMBL4267927) Inhibition of recombinant HIV1 reverse transcriptase K103N mutant
- ChEMBL_1795811 (CHEMBL4267928) Inhibition of recombinant HIV1 reverse transcriptase Y181C mutant
- ChEMBL_1795812 (CHEMBL4267929) Inhibition of recombinant HIV1 reverse transcriptase Y188L mutant
- ChEMBL_1798887 (CHEMBL4271179) Inhibition of EGFR T790M/L858R mutant (unknown origin)
- ChEMBL_1815520 (CHEMBL4315094) Inhibition of recombinant FLT3 ITD mutant (unknown origin)
- ChEMBL_1852132 (CHEMBL4352756) ATP competitive inhibition of human ABL T315I mutant
- ChEMBL_1852138 (CHEMBL4352762) ATP competitive inhibition of human Flt3 D835Y mutant
- ChEMBL_1859747 (CHEMBL4360603) Inhibition of Staphylococcus aureus DNA gyrase S84L mutant
- ChEMBL_1867089 (CHEMBL4368064) Corrector activity at CFTR F508del mutant (unknown origin)
- ChEMBL_1871094 (CHEMBL4372261) Inhibition of parkinson's patient-derived LRRK2 G2019S mutant
- ChEMBL_1932672 (CHEMBL4478324) Binding affinity to FLT3 ITD mutant (unknown origin)
- ChEMBL_1932673 (CHEMBL4478325) Binding affinity to FLT3 D835H mutant (unknown origin)
- ChEMBL_1932674 (CHEMBL4478326) Binding affinity to FLT3 D835Y mutant (unknown origin)
- ChEMBL_1933151 (CHEMBL4478803) Inhibition of HCV genotype 1a NS5B L419M mutant
- ChEMBL_1933152 (CHEMBL4478804) Inhibition of HCV genotype 1a NS5B L419I mutant
- ChEMBL_1933153 (CHEMBL4478805) Inhibition of HCV genotype 1a NS5B M423I mutant
- ChEMBL_1933154 (CHEMBL4478806) Inhibition of HCV genotype 1a NS5B M423T mutant
- ChEMBL_1933155 (CHEMBL4478807) Inhibition of HCV genotype 1a NS5B M423V mutant
- ChEMBL_1989762 (CHEMBL4623497) Inhibition of human BRAF V600E mutant by ELISA
- ChEMBL_2014795 (CHEMBL4668373) Inhibition of BCR-ABL T315I mutant (unknown origin)
- ChEMBL_2084911 (CHEMBL4766174) Inhibition of human recombinant B-RAF V600E mutant
- ChEMBL_209302 (CHEMBL811757) Inhibitory activity against mutant Plasmodium falciparum DHFR-TS
- ChEMBL_209440 (CHEMBL814296) Inhibitory activity against mutant Plasmodium falciparum DHFR-TS
- ChEMBL_2149932 (CHEMBL5034394) Binding affinity to ALK G1202R mutant (unknown origin)
- ChEMBL_2195570 (CHEMBL5107930) Inhibition of HIV-1 reverse transcriptase Y181C mutant
- ChEMBL_2201523 (CHEMBL5114231) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2201794 (CHEMBL5114502) Inhibition of EGFR del19/T790M mutant (unknown origin)
- ChEMBL_2201795 (CHEMBL5114503) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2213962 (CHEMBL5127094) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2260199 (CHEMBL5215210) Inhibition of EGFR L858R/T790M mutant (unknown origin)
- ChEMBL_2265031 Inhibition of EGFR L858R/T790M double mutant (unknown origin)
- ChEMBL_2271130 Binding affinity to B-Raf V600E mutant (unknown origin)
- ChEMBL_2271438 Inhibition of Abl T315I mutant in mouse BaF3 cells
- ChEMBL_2277135 Inhibition of HIV-1 integrase G140S/Q148K double mutant
- ChEMBL_2283197 Inhibition of EGFR L858R T790M/C797S mutant (unknown origin)
- ChEMBL_2294504 Inhibition of EGFR L858R/T790M/C797S mutant (unknown origin)
- ChEMBL_2319006 Inhibition of JAK2 V617F mutant in human SET2 cells
- ChEMBL_2319007 Inhibition of JAK2 V617F mutant in mouse BaF3 cells
- ChEMBL_2320999 Inhibition of EGFR del19 mutant in human HCC827 cells
- ChEMBL_2321233 Inhibition of influenza A virus H1N1 neuraminidase H274Y mutant
- ChEMBL_2326879 Inhibition of EGFR L858R/T790M/C797S mutant (unknown origin)
- ChEMBL_2356289 Inhibition of EGFR-D770-N771insNPG mutant (unknown origin) phosphorylation
- ChEMBL_2359697 Binding affinity to FLT3 ITD/D835V mutant (unknown origin)
- ChEMBL_2359698 Binding affinity to FLT3 ITD/F691L mutant (unknown origin)
- ChEMBL_2368831 Inhibition of His-tagged human recombinant TRKA G595R mutant
- ChEMBL_2368832 Inhibition of His-tagged human recombinant TRKA G667C mutant
- ChEMBL_2368834 Inhibition of His-tagged human recombinant TRKC G623R mutant
- ChEMBL_2379332 Inhibition of HIV-1 integrase G140S/Q148K double mutant
- ChEMBL_2379334 Inhibition of HIV-1 integrase DTG-resistant R263K mutant
- ChEMBL_2427239 Inhibition of FGFR4 C552A/C477A double mutant (unknown origin)
- ChEMBL_2434288 Inhibition of FLT3 ITD mutant in mouse BaF3 cells
- ChEMBL_2444179 Inhibition of KRAS G12C mutant in human HCC1171 cells
- ChEMBL_2455774 Inhibition of influenza A virus H1N1 neuraminidase H274Y mutant
- ChEMBL_2472966 Inhibition of EGFR L858R/T790M/C797S mutant (unknown origin)
- ChEMBL_2476283 Inhibition of EGFR L858R/T790M double mutant (unknown origin)
- ChEMBL_2476959 Inhibition of EGFR C797S/T790M/L858R mutant (unknown origin)
- ChEMBL_2476960 Inhibition of EGFR C797S/T790M/Del19 mutant (unknown origin)
- ChEMBL_2482008 Inhibition of EGFR Del19/T790M/C797S mutant (unknown origin)
- ChEMBL_2486813 Inhibition of GDP bound KRAS G12V mutant (unknown origin)
- ChEMBL_2500351 Inhibition of His-tagged human recombinant TRKC G623R mutant
- ChEMBL_2503171 Inhibition of EGFR Del19/T790M/C797S mutant (unknown origin)
- ChEMBL_2503172 Inhibition of EGFR L858R/T790M/C797S mutant (unknown origin)
- ChEMBL_2504431 Inhibition of Influenza A virus H5N1 neuraminidase H274Y mutant
- ChEMBL_302218 (CHEMBL826991) Dissociation constant against T4 lysozyme mutant L99A/M102Q
- ChEMBL_350973 (CHEMBL858908) Binding affinity to human BK1 receptor E273 mutant
- ChEMBL_350974 (CHEMBL858909) Binding affinity to human BK1 receptor D291 mutant
- ChEMBL_350975 (CHEMBL858910) Binding affinity to human BK1 receptor Q295 mutant
- ChEMBL_350976 (CHEMBL858911) Binding affinity to human BK1 receptor N298 mutant
- ChEMBL_365383 (CHEMBL870313) Inhibition of dimerization of HIV1 protease D30N mutant
- ChEMBL_365384 (CHEMBL870314) Inhibition of dimerization of HIV1 protease 150V mutant
- ChEMBL_365385 (CHEMBL870315) Inhibition of dimerization of HIV1 protease V82A mutant
- ChEMBL_478364 (CHEMBL931316) Inhibition of Escherichia coli LpxC Q202W/G210S mutant
- ChEMBL_478367 (CHEMBL931319) Inhibition of Rhizobium leguminosarum LpxC W206Q/S214G mutant
- ChEMBL_594352 (CHEMBL1048550) Inhibition of HIV1 protease L63P, V82T, I84V mutant
- ChEMBL_601741 (CHEMBL1041648) Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant
- ChEMBL_607109 (CHEMBL1066707) Inhibition of Staphylococcus aureus DNA gyrase Ser84Leu mutant
- ChEMBL_712676 (CHEMBL1660419) Inhibition of HIV1 Reverse transcriptase P119S/T165A mutant
- ChEMBL_712677 (CHEMBL1660420) Inhibition of HIV1 Reverse transcriptase P119S/M184V mutant
- ChEMBL_740706 (CHEMBL1763768) Inhibition of HTLV-1 recombinant protease L40I mutant
- ChEMBL_756687 (CHEMBL1805152) Inhibition of HIV1 free reverse transcriptase K103N mutant
- ChEMBL_756688 (CHEMBL1805153) Inhibition of HIV1 free reverse transcriptase L100I mutant
- ChEMBL_756689 (CHEMBL1805154) Inhibition of HIV1 free reverse transcriptase V179D mutant
- ChEMBL_766559 (CHEMBL1827040) Inhibition of full length B-Raf V600E mutant
- ChEMBL_798334 (CHEMBL1943822) Inhibition of full-length B-Raf V600E mutant
- ChEMBL_80426 (CHEMBL692910) Inhibitory concentration against TL3-resistant HIV(F53L) mutant
- ChEMBL_80550 (CHEMBL694593) Inhibitory concentration against drug-resistant HIV(G48V) mutant
- ChEMBL_80553 (CHEMBL694596) Inhibitory concentration against TL3-resistant HIV(L241) mutant
- ChEMBL_80556 (CHEMBL694107) Inhibitory concentration against TL3-resistant HIV(L63P) mutant
- ChEMBL_80559 (CHEMBL694110) Inhibitory concentration against TL3-resistant HIV(M461) mutant
- ChEMBL_80562 (CHEMBL694113) Inhibitory concentration against TL3-resistant HIV(V771) mutant
- ChEMBL_80565 (CHEMBL694116) Inhibitory concentration against TL3-resistant HIV(V82A) mutant
- ChEMBL_80568 (CHEMBL694119) Inhibitory concentration against drug-resistant HIV(V82F) mutant
- ChEMBL_818206 (CHEMBL2034076) Inhibition of full-length B-raf V600E mutant
- ChEMBL_851329 (CHEMBL2156036) Inhibition of full length B-Raf V600E mutant
- ChEMBL_958292 (CHEMBL2384766) Inhibition of Staphylococcus aureus DNA gyrase D83N mutant
- ChEMBL_958293 (CHEMBL2384767) Inhibition of Staphylococcus aureus DNA gyrase M121K mutant
- ChEMBL_1543930 (CHEMBL3750038) Inhibition of human recombinant PLK1 using recombinant dephosphorylated bovine alpha- casein/synuclein as substrate after 30 mins by scintillation counting analysis in presence of gamma-32P-ATP
- ChEMBL_1543931 (CHEMBL3750039) Inhibition of human recombinant PLK3 using recombinant dephosphorylated bovine alpha- casein/synuclein as substrate after 30 mins by scintillation counting analysis in presence of gamma-32P-ATP
- ChEMBL_1543932 (CHEMBL3750040) Inhibition of human recombinant PLK4 using recombinant dephosphorylated bovine alpha- casein/synuclein as substrate after 30 mins by scintillation counting analysis in presence of gamma-32P-ATP



