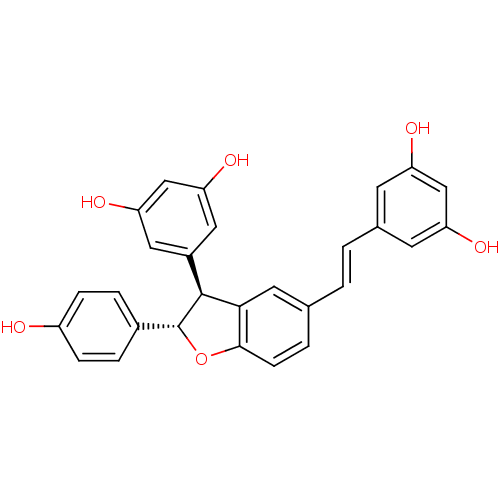
 CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer
CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer Azo-Resveratrol CHEMBL2208146 BDBM50486146
Azo-Resveratrol CHEMBL2208146 BDBM50486146 CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate
CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate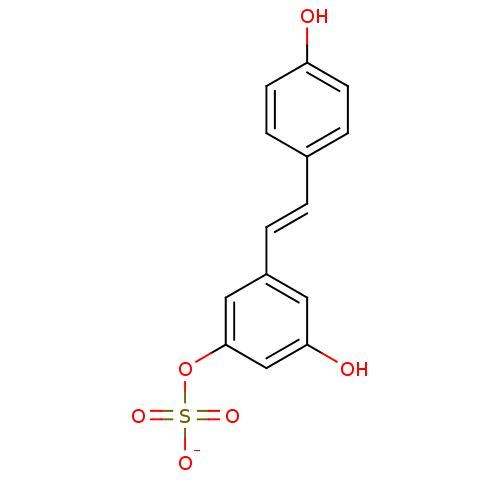 Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816
Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816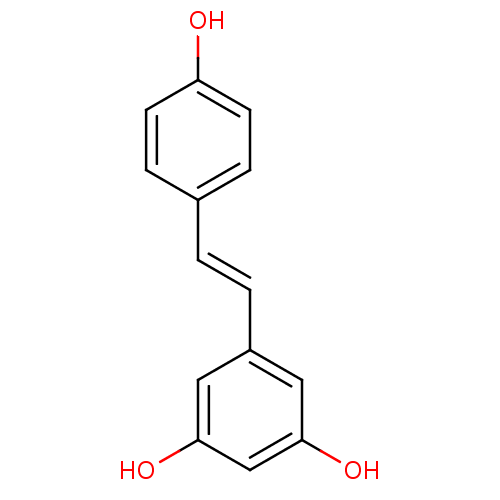 BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f
BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f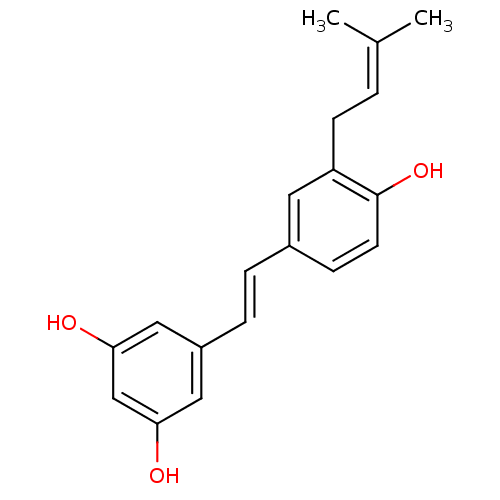 3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145
3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145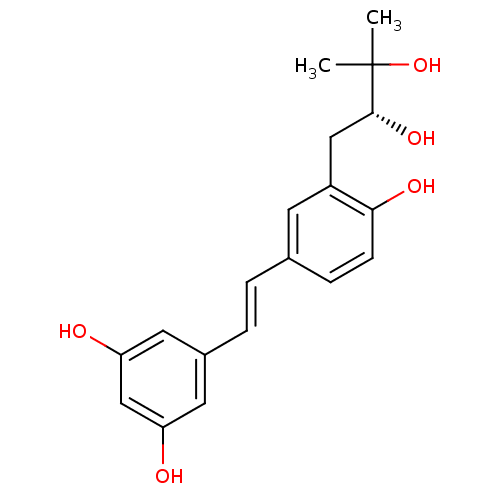 BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol
BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol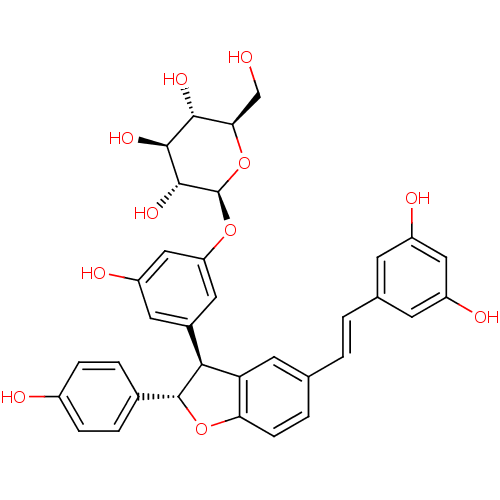 CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812
CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812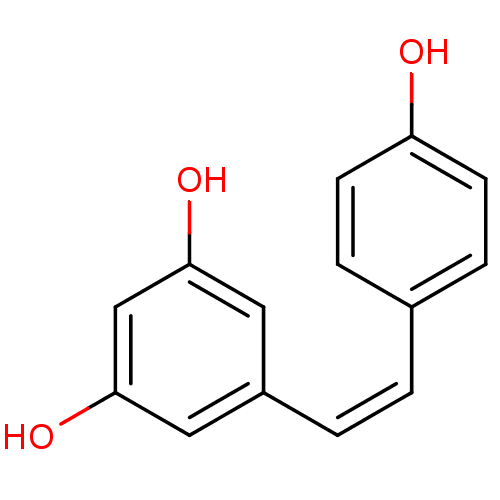 5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol
5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol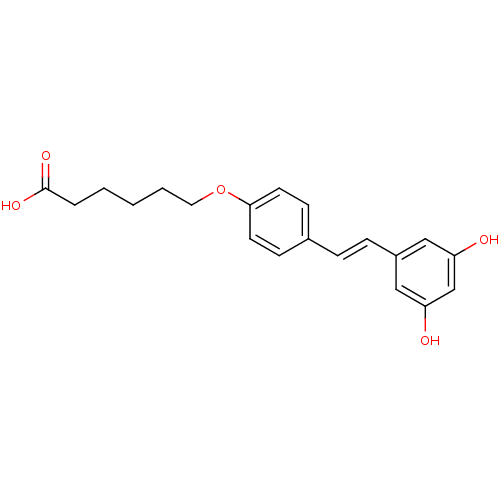 Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid
Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid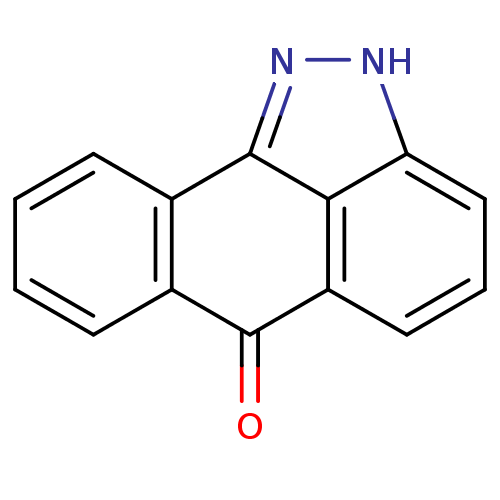 JMC517015 Compound 2 dibenzo[cd,g]indazol-6(2H)-one BDBM16018 SP 600125 SP-600125 ChemBiol10705 Compound 4 14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexadeca-1(15),2(7),3,5,9(16),10,12-heptaen-8-one cid_8515 CHEMBL7064 SP600125
JMC517015 Compound 2 dibenzo[cd,g]indazol-6(2H)-one BDBM16018 SP 600125 SP-600125 ChemBiol10705 Compound 4 14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexadeca-1(15),2(7),3,5,9(16),10,12-heptaen-8-one cid_8515 CHEMBL7064 SP600125
- Yokoyama, T; Kusaka, K; Mizuguchi, M; Nabeshima, Y; Fujiwara, S Resveratrol Derivatives Inhibit Transthyretin Fibrillization: Structural Insights into the Interactions between Resveratrol Derivatives and Transthyretin. J Med Chem 66: 15511-15523 (2023)
- Grau, L; Soucek, R; Pujol, MD Resveratrol derivatives: Synthesis and their biological activities. Eur J Med Chem 246: (2023)
- Cichewicz, RH; Kouzi, SA; Hamann, MT Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea. J Nat Prod 63: 29-33 (2000)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimizing thiadiazole analogues of resveratrol versus three chemopreventive targets. Bioorg Med Chem 20: 510-20 (2011)
- Nalli, M; Ortar, G; Moriello, AS; Morera, E; Di Marzo, V; De Petrocellis, L TRPA1 channels as targets for resveratrol and related stilbenoids. Bioorg Med Chem Lett 26: 899-902 (2016)
- Kusakabe, K; Ide, N; Daigo, Y; Tachibana, Y; Itoh, T; Yamamoto, T; Hashizume, H; Hato, Y; Higashino, K; Okano, Y; Sato, Y; Inoue, M; Iguchi, M; Kanazawa, T; Ishioka, Y; Dohi, K; Kido, Y; Sakamoto, S; Yasuo, K; Maeda, M; Higaki, M; Ueda, K; Yoshizawa, H; Baba, Y; Shiota, T; Murai, H; Nakamura, Y Indazole-based potent and cell-active Mps1 kinase inhibitors: rational design from pan-kinase inhibitor anthrapyrazolone (SP600125). J Med Chem 56: 4343-56 (2013)
- Pettit, GR; Grealish, MP; Jung, MK; Hamel, E; Pettit, RK; Chapuis, JC; Schmidt, JM Antineoplastic agents. 465. Structural modification of resveratrol: sodium resverastatin phosphate. J Med Chem 45: 2534-42 (2002)
- Cardullo, N; Spatafora, C; Musso, N; Barresi, V; Condorelli, D; Tringali, C Resveratrol-Related Polymethoxystilbene Glycosides: Synthesis, Antiproliferative Activity, and Glycosidase Inhibition. J Nat Prod 78: 2675-83 (2015)
- Fantacuzzi, M; Amoroso, R; Carradori, S; De Filippis, B Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur J Med Chem 233: (2022)
- Hoshino, J; Park, EJ; Kondratyuk, TP; Marler, L; Pezzuto, JM; van Breemen, RB; Mo, S; Li, Y; Cushman, M Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem 53: 5033-43 (2010)
- Das, J; Pany, S; Majhi, A Chemical modifications of resveratrol for improved protein kinase C alpha activity. Bioorg Med Chem 19: 5321-33 (2011)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimization of the aromatase inhibitory activities of pyridylthiazole analogues of resveratrol. Bioorg Med Chem 20: 2427-34 (2012)
- Cheng, G; Xu, P; Zhang, M; Chen, J; Sheng, R; Ma, Y Resveratrol-maltol hybrids as multi-target-directed agents for Alzheimer's disease. Bioorg Med Chem 26: 5759-5765 (2018)
- Xu, P; Zhang, M; Sheng, R; Ma, Y Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ Eur J Med Chem 127: 174-186 (2017)
- Tang, YW; Shi, CJ; Yang, HL; Cai, P; Liu, QH; Yang, XL; Kong, LY; Wang, XB Synthesis and evaluation of isoprenylation-resveratrol dimer derivatives against Alzheimer's disease. Eur J Med Chem 163: 307-319 (2019)
- Aldawsari, FS; Aguiar, RP; Wiirzler, LA; Aguayo-Ortiz, R; Aljuhani, N; Cuman, RK; Medina-Franco, JL; Siraki, AG; Velázquez-Martínez, CA Anti-inflammatory and antioxidant properties of a novel resveratrol-salicylate hybrid analog. Bioorg Med Chem Lett 26: 1411-5 (2016)
- Vilar, S; Quezada, E; Santana, L; Uriarte, E; Yánez, M; Fraiz, N; Alcaide, C; Cano, E; Orallo, F Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids. Bioorg Med Chem Lett 16: 257-61 (2005)
- Jerábek, J; Uliassi, E; Guidotti, L; Korábecný, J; Soukup, O; Sepsova, V; Hrabinova, M; Kuca, K; Bartolini, M; Peña-Altamira, LE; Petralla, S; Monti, B; Roberti, M; Bolognesi, ML Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer's disease. Eur J Med Chem 127: 250-262 (2017)
- Ahmadi, R; Ebrahimzadeh, MA Resveratrol - A comprehensive review of recent advances in anticancer drug design and development. Eur J Med Chem 200: (2020)
- Jiang, YL Design, synthesis and spectroscopic studies of resveratrol aliphatic acid ligands of human serum albumin. Bioorg Med Chem 16: 6406-14 (2008)
- Duan, YC; Guan, YY; Zhai, XY; Ding, LN; Qin, WP; Shen, DD; Liu, XQ; Sun, XD; Zheng, YC; Liu, HM Discovery of resveratrol derivatives as novel LSD1 inhibitors: Design, synthesis and their biological evaluation. Eur J Med Chem 126: 246-258 (2017)
- Menezes, JCJMDS; Diederich, MF Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur J Med Chem 182: (2019)
- Song, YM; Ha, YM; Kim, JA; Chung, KW; Uehara, Y; Lee, KJ; Chun, P; Byun, Y; Chung, HY; Moon, HR Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 22: 7451-5 (2012)
- Nakao, S; Mabuchi, M; Wang, S; Kogure, Y; Shimizu, T; Noguchi, K; Tanaka, A; Dai, Y Synthesis of resveratrol derivatives as new analgesic drugs through desensitization of the TRPA1 receptor. Bioorg Med Chem Lett 27: 3167-3172 (2017)
- Hao, XD; Chang, J; Qin, BY; Zhong, C; Chu, ZB; Huang, J; Zhou, WJ; Sun, X Synthesis, estrogenic activity, and anti-osteoporosis effects in ovariectomized rats of resveratrol oligomer derivatives. Eur J Med Chem 102: 26-38 (2015)
- Lu, C; Guo, Y; Yan, J; Luo, Z; Luo, HB; Yan, M; Huang, L; Li, X Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer's disease. J Med Chem 56: 5843-59 (2014)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimization of thiazole analogues of resveratrol for induction of NAD(P)H:quinone reductase 1 (QR1). Bioorg Med Chem 20: 7030-9 (2012)
- Yao, RS; Lu, XQ; Guan, QX; Zheng, L; Lu, X; Ruan, BF Synthesis and biological evaluation of some novel resveratrol amide derivatives as potential anti-tumor agents. Eur J Med Chem 62: 222-31 (2013)
- Lee, I; Choe, YS; Choi, JY; Lee, KH; Kim, BT Synthesis and evaluation of ¹⁸F-labeled styryltriazole and resveratrol derivatives for β-amyloid plaque imaging. J Med Chem 55: 883-92 (2012)
- Samaradivakara, SP; Samarasekera, R; Handunnetti, SM; Weerasena, OVDSJ; Al-Hamashi, AA; Slama, JT; Taylor, WR; Alhadidi, Q; Shah, ZA; Perera, L; Tillekeratne, LMV A Bioactive Resveratrol Trimer from the Stem Bark of the Sri Lankan Endemic Plant Vateria copallifera. J Nat Prod 81: 1693-1700 (2018)
- St John, SE; Jensen, KC; Kang, S; Chen, Y; Calamini, B; Mesecar, AD; Lipton, MA Design, synthesis, biological and structural evaluation of functionalized resveratrol analogues as inhibitors of quinone reductase 2. Bioorg Med Chem 21: 6022-37 (2013)
- Herrera-Arozamena, C; Estrada-Valencia, M; López-Caballero, P; Pérez, C; Morales-García, JA; Pérez-Castillo, A; Sastre, ED; Fernández-Mendívil, C; Duarte, P; Michalska, P; Lombardía, J; Senar, S; León, R; López, MG; Rodríguez-Franco, MI Resveratrol-Based MTDLs to Stimulate Defensive and Regenerative Pathways and Block Early Events in Neurodegenerative Cascades. J Med Chem 65: 4727-4751 (2022)
- de Medina, P; Casper, R; Savouret, JF; Poirot, M Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem 48: 287-91 (2005)
- Resveratrol-derived inhibitors of the E3 ubiquitin ligase PELI1 inhibit the metastasis of triple-negative breast cancer.
- Ruan, BF; Cheng, HJ; Ren, J; Li, HL; Guo, LL; Zhang, XX; Liao, C Novel 2H-chromen-2-one derivatives of resveratrol: Design, synthesis, modeling and use as human monoamine oxidase inhibitors. Eur J Med Chem 103: 185-90 (2015)
- Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem 18: 5352-66 (2010)
- Mikstacka, R; Rimando, AM; Dutkiewicz, Z; Stefanski, T; Sobiak, S Design, synthesis and evaluation of the inhibitory selectivity of novel trans-resveratrol analogues on human recombinant CYP1A1, CYP1A2 and CYP1B1. Bioorg Med Chem 20: 5117-26 (2012)
- Kang, SS; Cuendet, M; Endringer, DC; Croy, VL; Pezzuto, JM; Lipton, MA Synthesis and biological evaluation of a library of resveratrol analogues as inhibitors of COX-1, COX-2 and NF-kappaB. Bioorg Med Chem 17: 1044-54 (2009)
- Yang, X; Qiang, X; Li, Y; Luo, L; Xu, R; Zheng, Y; Cao, Z; Tan, Z; Deng, Y Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer's disease. Bioorg Chem 71: 305-314 (2017)
 CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer
CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer Azo-Resveratrol CHEMBL2208146 BDBM50486146
Azo-Resveratrol CHEMBL2208146 BDBM50486146 CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate
CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816
Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816 BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f
BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f 3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145
3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145 BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol
BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812
CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812 5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol
5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid
Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid JMC517015 Compound 2 dibenzo[cd,g]indazol-6(2H)-one BDBM16018 SP 600125 SP-600125 ChemBiol10705 Compound 4 14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexadeca-1(15),2(7),3,5,9(16),10,12-heptaen-8-one cid_8515 CHEMBL7064 SP600125
JMC517015 Compound 2 dibenzo[cd,g]indazol-6(2H)-one BDBM16018 SP 600125 SP-600125 ChemBiol10705 Compound 4 14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexadeca-1(15),2(7),3,5,9(16),10,12-heptaen-8-one cid_8515 CHEMBL7064 SP600125