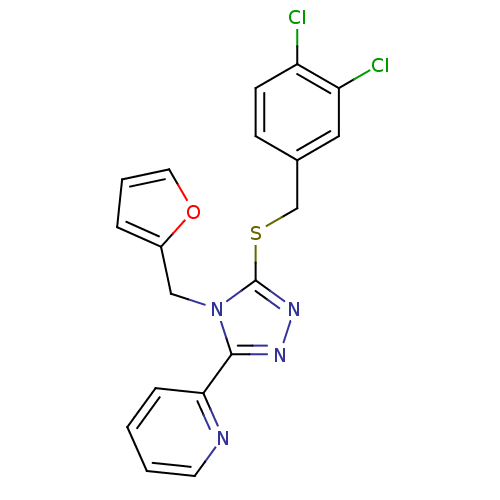
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataKi: 40nMAssay Description:Agonist activity at human kappa opioid receptor expressed in CHO cell membranes by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataIC50: >3.20E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 347nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics Source Affiliation: Sanford-Burnham Medical Research Institute Network: NIH Molecular Lib...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 867nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 870nMAssay Description:Inhibition of kappa opioid receptor (unknown origin) assessed as increase in beta arrestin 2 recruitmentMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 8.99E+3nMAssay Description:Inhibition of rat TRPA1 at a holding potential of 15 mV measured after 1 min by whole-cell manual patch clamp electrophysiology assayMore data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 870nMAssay Description:Agonist activity at human kappa opioid receptor expressed in human U2OS cells co-transfected with EFC and beta-arrestin-2 assessed as increase in bet...More data for this Ligand-Target Pair
TargetKappa-type opioid receptor(Homo sapiens (Human))
The Scripps Research Institute
Curated by ChEMBL
The Scripps Research Institute
Curated by ChEMBL
Affinity DataEC50: 1.40E+5nMAssay Description:Inhibition of kappa opioid receptor (unknown origin) assessed as increase in beta arrestin 2 recruitment relative to controlMore data for this Ligand-Target Pair